All the noble gases are colourless and tasteless monoatomic gases. In general, noble gases are least reactive and their inertness to chemical reactivity is attributed to the following reasons. Reactive and their inertness to chemical reactivity is attributed to the following reasons.
(i)Except helium, all have completely filled ns2np6 electronic configuration in their valence shells.
(ii)All have high ionisation enthalpy and more positive electron gain enthalpy.
However, a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen have been synthesized under different conditions and fluorides of xenon have been used as an oxidising agent and a fluorinating agent in many of the chemical reactions
Select the correct statement.
Neon does not form clatherate compound with para-quinol
Noble gases are sparingly soluble in water
Helium is a non-inflammable and light gas, therefore, it is used in filling balloons for meteorological observations.
All of these
The correct Answer is:D
To solve the question regarding the properties and reactivity of noble gases, we will analyze the statements provided and determine their correctness step by step.
Step 1: Understanding Noble Gases
Noble gases are known for being colorless, tasteless, and monoatomic. They are generally considered to be the least reactive elements in the periodic table.
Hint: Remember the characteristics of noble gases that contribute to their inertness.
Step 2: Analyzing the Electronic Configuration
The electronic configuration of noble gases (except helium) is ns2np6, which means their valence shells are completely filled. This full valence shell is a key reason for their low reactivity.
Hint: Consider how a full valence shell influences reactivity.
Step 3: Ionization Enthalpy and Electron Gain Enthalpy
Noble gases have high ionization enthalpy and positive electron gain enthalpy. This means that it requires a lot of energy to remove an electron from them, and they do not easily gain electrons. This contributes to their inertness.
Hint: Think about how ionization energy and electron affinity relate to chemical reactivity.
Step 4: Compounds of Xenon
Although noble gases are generally inert, xenon can form compounds with highly electronegative elements like fluorine and oxygen. This shows that under certain conditions, noble gases can exhibit some reactivity.
Hint: Recall the exceptions to the general behavior of noble gases.
Step 5: Evaluating the Statements
Now, let's evaluate the statements provided in the question:
1. Neon does not clathrate with para-quinone. - This statement is true. Neon is less likely to form clathrate compounds due to its small size and low polarizability.
2. Noble gases are sparingly soluble in water. - This statement is also true. Noble gases have low solubility in water due to their non-polar nature.
3. Helium is non-inflammable and a light gas; therefore, it is used for filling balloons for meteorological observation. - This statement is true as well. Helium is safe and lighter than air, making it suitable for balloons.
Step 6: Conclusion
Since all the statements provided are true, the correct answer to the question is option D: All of these.
Final Answer: Option D: All of these.
Noble gases are known for being colorless, tasteless, and monoatomic. They are generally considered to be the least reactive elements in the periodic table.
The electronic configuration of noble gases (except helium) is ns2np6, which means their valence shells are completely filled. This full valence shell is a key reason for their low reactivity.
Noble gases have high ionization enthalpy and positive electron gain enthalpy. This means that it requires a lot of energy to remove an electron from them, and they do not easily gain electrons. This contributes to their inertness.
Although noble gases are generally inert, xenon can form compounds with highly electronegative elements like fluorine and oxygen. This shows that under certain conditions, noble gases can exhibit some reactivity.
Now, let's evaluate the statements provided in the question:
2. Noble gases are sparingly soluble in water. - This statement is also true. Noble gases have low solubility in water due to their non-polar nature.
3. Helium is non-inflammable and a light gas; therefore, it is used for filling balloons for meteorological observation. - This statement is true as well. Helium is safe and lighter than air, making it suitable for balloons.
Since all the statements provided are true, the correct answer to the question is option D: All of these.
Topper's Solved these Questions
- P BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - P BLOCK ELEMENTSEXERCISE - PART -II
23 Videos
- P BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - P BLOCK ELEMENTSEXERCISE - Exercise 3 part-3
28 Videos
- NUCLEAR CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - NUCLEAR CHEMISTRYEXERCISE - STAGE-II
1 Video
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)BOOK - RESONANCE ENGLISHCHAPTER - P-BLOCK ELEMENT (BORON AND CARBON FAMILY)EXERCISE - PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
8 Videos
Similar Questions
All the noble gases are colourless and tasteless monoatomic gases. In general, noble gases are least reactive and their inertness to chemical reactivity is attributed to the following reasons. Reactive and their inertness to chemical reactivity is attributed to the following reasons.
(i)Except helium, all have completely filled ns2np6 electronic configuration in their valence shells.
(ii)All have high ionisation enthalpy and more positive electron gain enthalpy.
However, a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen have been synthesized under different conditions and fluorides of xenon have been used as an oxidising agent and a fluorinating agent in many of the chemical reactions
The correct order of the abundance of various noble gases in air is :
View SolutionInert gases are least reactive due to :-
View SolutionWhy do noble gases have positive electron gain enthalpies?
View SolutionWhich shows the least chemical reactivity?
View SolutionWhich of the following is least reactive?
View SolutionState why noble gases are unreactive while atoms of elements other than noble gases are chemically reactive.
View SolutionXe is the most reactive noble gas
View SolutionStatement-1 : Argon is used in the laboratory for handling substances that are air-sensitive.
Statement-2 : Argon is inert towards chemical reactivity due to the completely filled valence shell electronic configuration, high ionization enthalpy and more positive electron gain enthalpy.
View SolutionGradual addition of electronic shells in the noble gases causes a decrease in their
View SolutionMatch the correct ionisation enthalpies and electron gain enthalpies of the following elements.
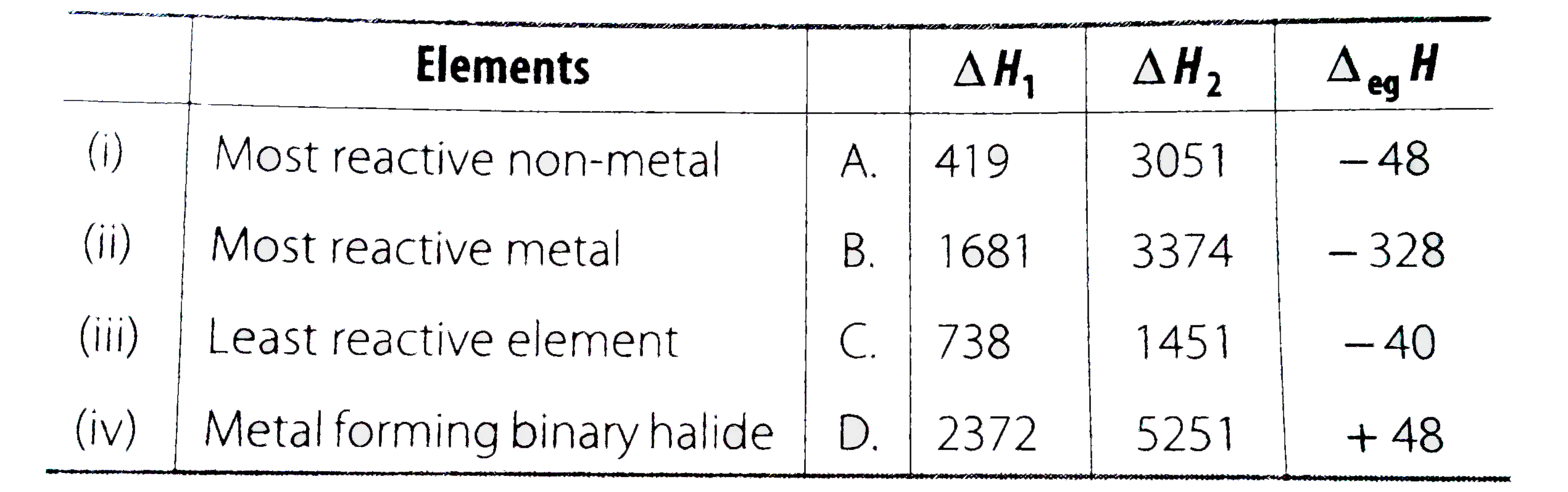
View Solution

