Hydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
The energy required to promote the ground state electron of H-atom to the first excited state is:
When an electron returns from a higher energy level to a lower energy level, energy is given out in the form of UV//Visible radiation.
The correct Answer is:10.2eV
To solve the problem of calculating the energy required to promote the ground state electron of a hydrogen atom to the first excited state, we can follow these steps:
Step 1: Understand the Energy Formula
The energy of a hydrogen-like atom can be expressed using the formula:
En=−RHZ2n2
where:
- RH is the Rydberg constant (approximately 13.6 eV for hydrogen),
- Z is the atomic number (for hydrogen, Z=1),
- n is the principal quantum number.
Step 2: Identify the Energy Levels
For hydrogen:
- The ground state corresponds to n1=1.
- The first excited state corresponds to n2=2.
Step 3: Calculate Energy for Ground State (n=1)
Using the formula for n1=1:
E1=−RH⋅1212=−RH
Substituting RH=13.6eV:
E1=−13.6eV
Step 4: Calculate Energy for First Excited State (n=2)
Using the formula for n2=2:
E2=−RH⋅1222=−RH4
Substituting RH=13.6eV:
E2=−13.64=−3.4eV
Step 5: Calculate the Energy Difference
The energy required to promote the electron from the ground state to the first excited state is given by the difference in energy:
ΔE=E2−E1
Substituting the values we calculated:
ΔE=(−3.4eV)−(−13.6eV)
ΔE=−3.4+13.6=10.2eV
Conclusion
The energy required to promote the ground state electron of the hydrogen atom to the first excited state is:
ΔE=10.2eV
The energy of a hydrogen-like atom can be expressed using the formula:
En=−RHZ2n2
where:
- RH is the Rydberg constant (approximately 13.6 eV for hydrogen),
- Z is the atomic number (for hydrogen, Z=1),
- n is the principal quantum number.
For hydrogen:
- The ground state corresponds to n1=1.
- The first excited state corresponds to n2=2.
Using the formula for n1=1:
E1=−RH⋅1212=−RH
Substituting RH=13.6eV:
E1=−13.6eV
Using the formula for n2=2:
E2=−RH⋅1222=−RH4
Substituting RH=13.6eV:
E2=−13.64=−3.4eV
The energy required to promote the electron from the ground state to the first excited state is given by the difference in energy:
ΔE=E2−E1
Substituting the values we calculated:
ΔE=(−3.4eV)−(−13.6eV)
ΔE=−3.4+13.6=10.2eV
The energy required to promote the ground state electron of the hydrogen atom to the first excited state is:
ΔE=10.2eV
समान प्रश्न
Hydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
The energy in Joule of an electron in the second orbit of H- atom is:
View SolutionHydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
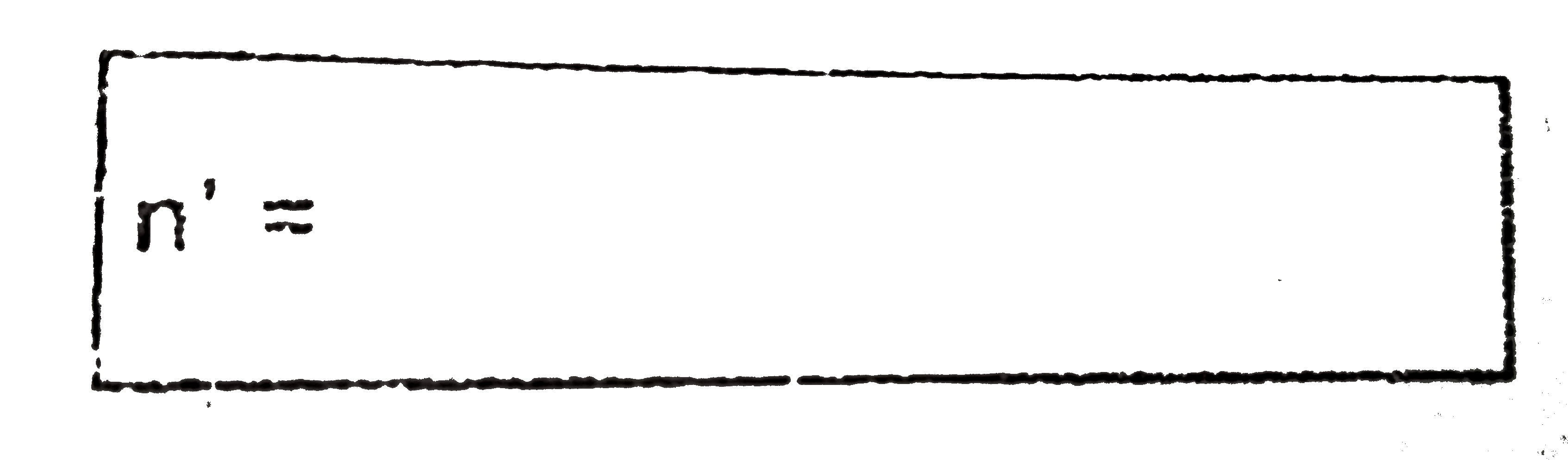
What is the principal quantum number, n' of the orbit of Be3 that has the same Bohr radius as that of ground state hydrogen atom ?
View Solution
ज्ञान जांच
the energy required to excite an electron in hydrogen atom to its first excited state is
A8.5eVB10.2eVC12.7eVD13.6eV
Hydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
Calculate the following :
(a) the kinetic energy (in eV) of an electron in the ground state of hydrogen atom.
(b) the potential energy (in eV) of an electron in the ground state of hydrogen atom.
View SolutionHydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
Calculate the wavelength of light (nm) for the electronic transition of H-atom from the first excited state to ground state.
In the model of hydrogen like atom put forward by Niels Bohr (1913) the electron orbits around the central nucleus. the bohr radius of nth orbit of a hydrogen-like species is given by
r=kn2Z " " where, k is constant
View SolutionHydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
The ratio of energy of an electron in the ground state Be3− ion to that of ground state H atom is:
The kinetic and potential energies of an electron in the H atoms are given as
K.E.=e24πε02r and P.E.=−14πε0e2r
View SolutionWhen an electron of hydrogen like atom jumps from a higher energy level to a lower energy level.
View SolutionIf the electron of a hydrogen atom is present in the first orbit. The total energy of the electrons is
View SolutionThe energy of the electron in the ground state of hydrogen atom is −13.6eV. Find the kinetic energy of electron in this state.
View SolutionThe speed of an electron in the orbit of hydrogen atom in the ground state is
View Solution
