Entropy is a state function and its value depends on two or three variable temperature (T), Pressure(P) and volume (V). Entropy change for an ideal gas having number of moles(n) can be determined by the following equation.
ΔS=2.303nCvlog(T2T1)+2.303nR log(V2V1)
ΔS=2.303nCPlog(T2T1)+2.303nR log(P1P2)
Since free energy change for a process or a chemical equation is a deciding factor of spontaneity , which can be obtained by using entropy change (ΔS) according to the expression, ΔG=ΔH−TΔS at a temperature T
What would be the entropy change involved in thermodynamic expansion of 2 moles of a gas from a volume of 5 L to a volume of 50 L at 25∘ C [Given R=8.3J/mole−K]
38.23 J/K mol
26.76 J/K
20 J/k
28.23 J/K
The correct Answer is:A
To find the entropy change involved in the thermodynamic expansion of 2 moles of a gas from a volume of 5 L to a volume of 50 L at 25°C, we can use the formula for entropy change for an ideal gas during an isothermal expansion:
ΔS=n⋅2.303⋅R⋅log(V2V1)
1. Identify the Variables:
- Number of moles, n=2 moles
- Initial volume, V1=5 L
- Final volume, V2=50 L
- Gas constant, R=8.3J/(mole⋅K)
- Temperature, T=25∘C=298K (not needed for this calculation since the process is isothermal)
2. Calculate the Volume Ratio:
V2V1=50L5L=10
3. Calculate the Logarithm:
log(V2V1)=log(10)=1
4. Substitute Values into the Entropy Change Formula:
ΔS=2⋅2.303⋅8.3⋅1
5. Perform the Calculation:
ΔS=2⋅2.303⋅8.3
=2⋅19.1159≈38.23J/K
Final Answer:
The entropy change involved in the thermodynamic expansion is approximately ΔS≈38.23J/K.
- Number of moles, n=2 moles
- Initial volume, V1=5 L
- Final volume, V2=50 L
- Gas constant, R=8.3J/(mole⋅K)
- Temperature, T=25∘C=298K (not needed for this calculation since the process is isothermal)
V2V1=50L5L=10
log(V2V1)=log(10)=1
ΔS=2⋅2.303⋅8.3⋅1
ΔS=2⋅2.303⋅8.3
=2⋅19.1159≈38.23J/K
The entropy change involved in the thermodynamic expansion is approximately ΔS≈38.23J/K.
Topper's Solved these Questions
- THERMODYNAMICSBook:RESONANCE ENGLISHChapter:THERMODYNAMICSExercise:exercise-3 Part:(I)
Explore 23 Videos
- THERMODYNAMICSBook:RESONANCE ENGLISHChapter:THERMODYNAMICSExercise:exercise-3 part-1 Advanced level Solutions
Explore 30 Videos
- THERMODYNAMICSBook:RESONANCE ENGLISHChapter:THERMODYNAMICSExercise:exercise-2 Part:(III): One or more than one options correct
Explore 8 Videos
- TEST SERIESBook:RESONANCE ENGLISHChapter:TEST SERIESExercise:CHEMISTRY
Explore 50 Videos
Similar Questions
Entropy is a state function and its value depends on two or three variable temperature (T), Pressure(P) and volume (V). Entropy change for an ideal gas having number of moles(n) can be determined by the following equation.
ΔS=2.303nCvlog(T2T1)+2.303nR log(V2V1)
ΔS=2.303nCPlog(T2T1)+2.303nR log(P1P2)
Since free energy change for a process or a chemical equation is a deciding factor of spontaneity , which can be obtained by using entropy change (ΔS) according to the expression, ΔG=ΔH−TΔS at a temperature T
An isobaric process having one mole of ideal gas has entropy change 23.03 J/K for the temperature range 27∘ C to 327∘ C . What would be the molar specific heat capacity (Cv)?
View SolutionEntropy is a state function and its value depends on two or three variable temperature (T), Pressure(P) and volume (V). Entropy change for an ideal gas having number of moles(n) can be determined by the following equation.
ΔS=2.303nCvlog(T2T1)+2.303nR log(V2V1)
ΔS=2.303nCPlog(T2T1)+2.303nR log(P1P2)
Since free energy change for a process or a chemical equation is a deciding factor of spontaneity , which can be obtained by using entropy change (ΔS) according to the expression, ΔG=ΔH−TΔS at a temperature T
For a reaction M2O(s)→2M(s)+12O2(g),ΔH=30KJ/mol and ΔS=0.07KJmo≤at1 atm. Calculate upto which temperature the reaction would not be spontaneous.
View SolutionIf Kc is not numerically equal to Kp, how can both of the following equations be valid?
ΔGɵ=−2.303RTlogKc,ΔGɵ=−2.303RTlogKp
View SolutionFor V versus T curves at constant pressure P1andP2 for an ideal gas are shown in the figure given below
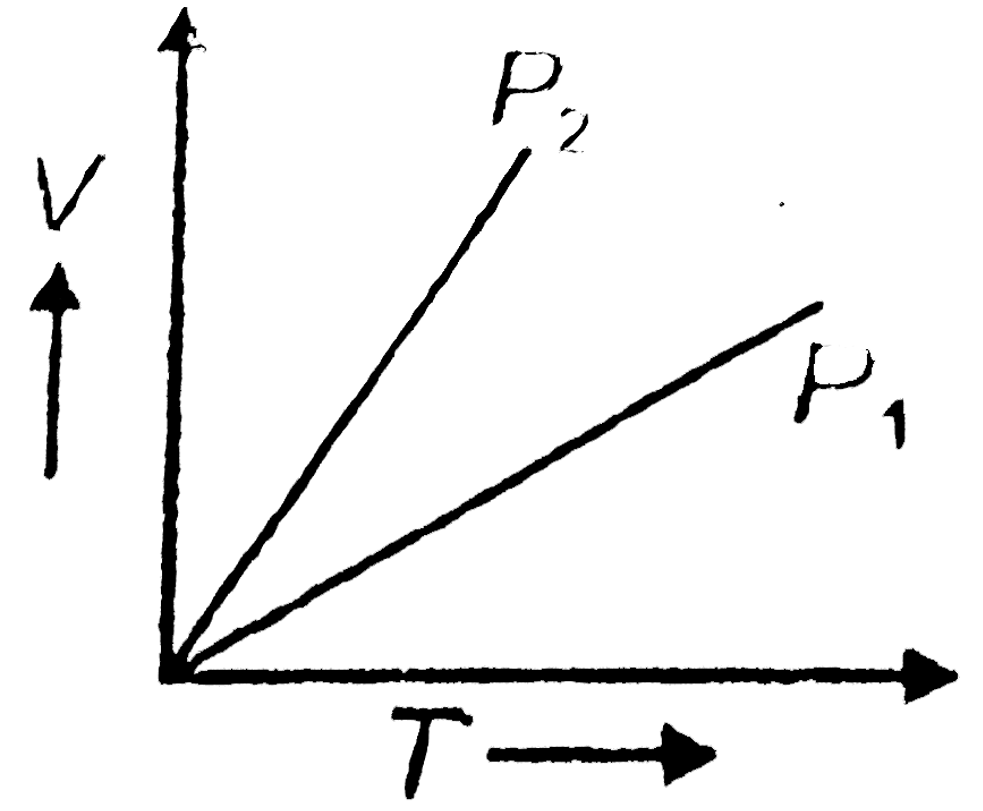
View SolutionV vs T curves at constant pressure P1 and P2 for an ideal gas are shown below
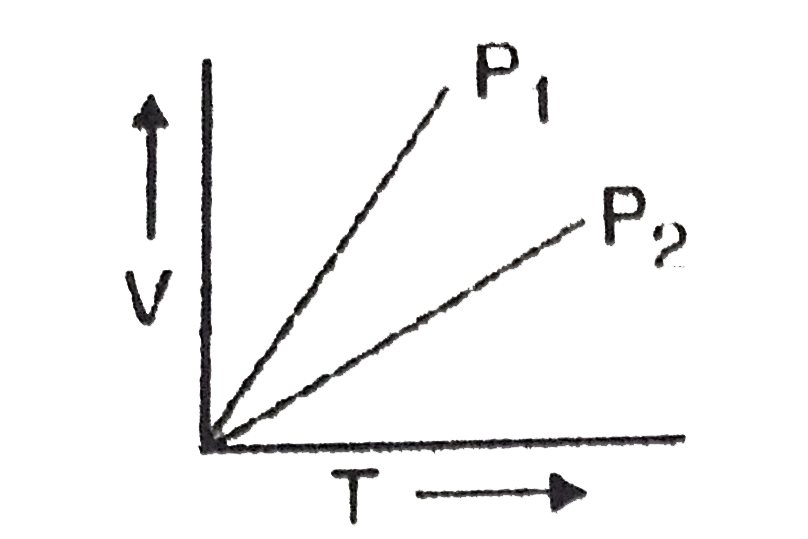
Which is correct ?
View SolutionFor a sample of n mole of a perfect gas at constant temperature T (K) when its volume is changed from V1 to V2 the entropy change is given by
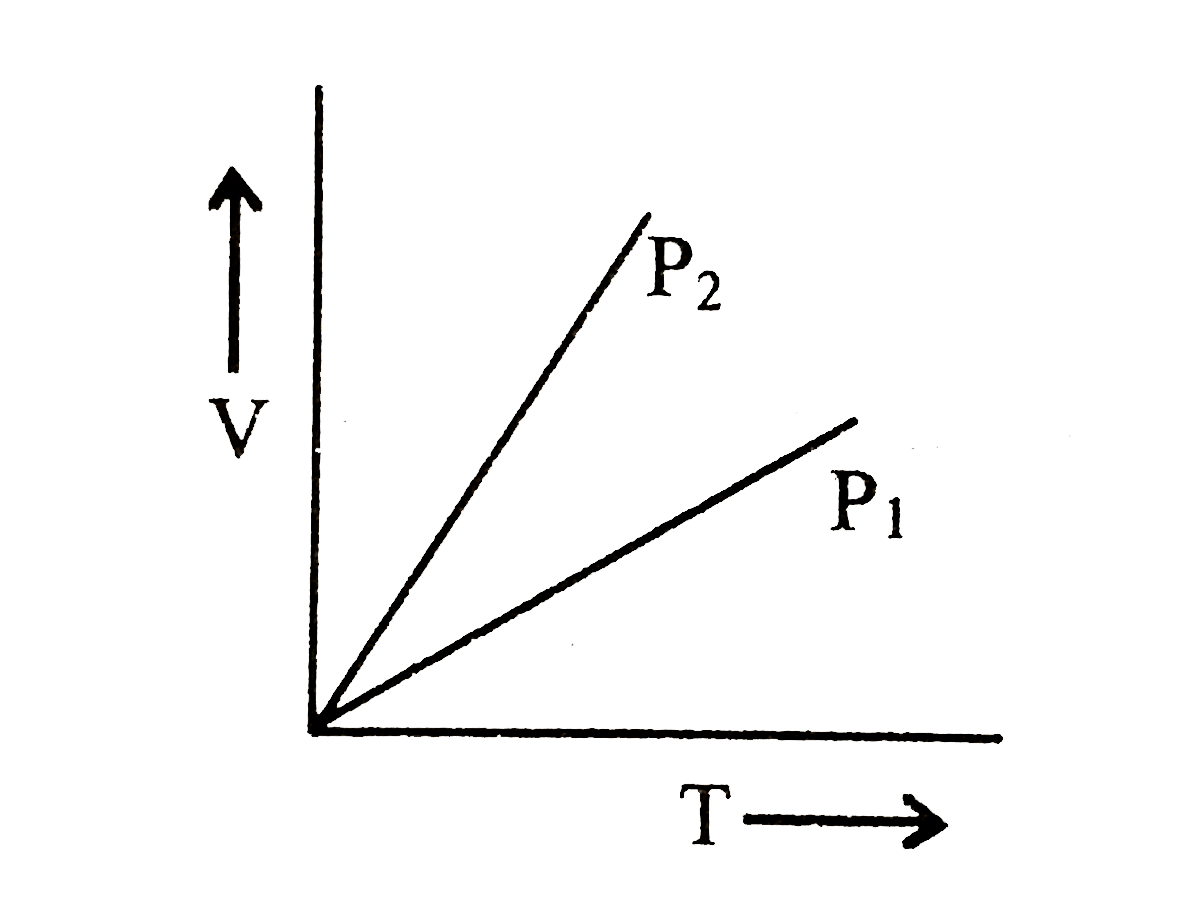
View SolutionFor V versus T curves at constant pressure P1andP2 for and ideal gas shown in figure-
View SolutionFigure shows volume - temperature (V-T) graph for same mass of an ideal gas at two different pressures P1and P2. Hence
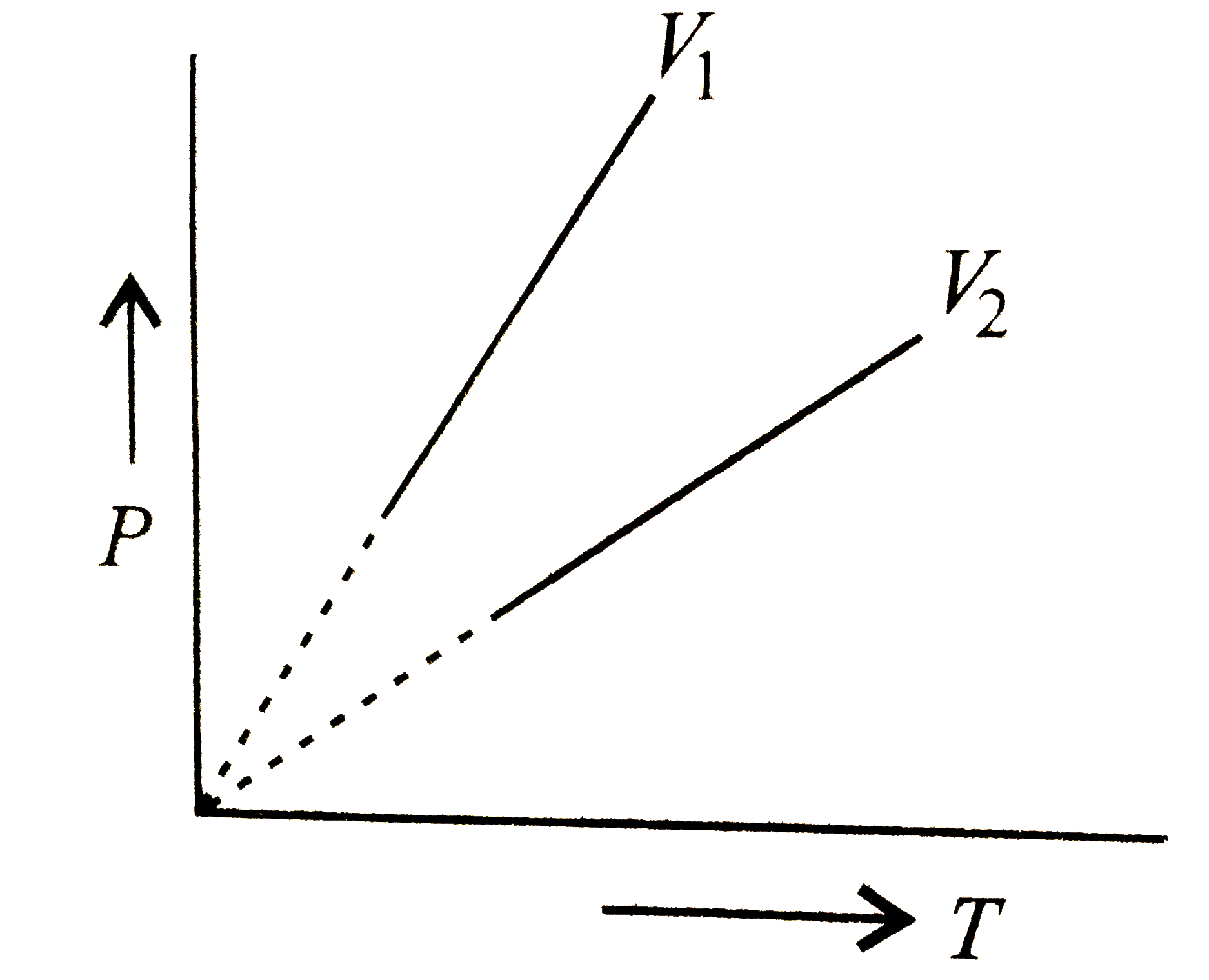
View SolutionA plot of P vs T for a given mass of gas at constant volume is a straight line . P vs T at constant volumes V1 and V2 for an ideal gas are shown below :
Which of the following is correct ?
View SolutionThe equation of state of a real gas is given by (P+aV2)(V−b)=RT
where P, V and T are pressure, volume and temperature resperature and R is the universal gas constant. The dimensions of the constant a in the above equation is
View Solution
