Which type of reaction MnO2−4show with acid , dilute -alkali or water.
It is unstable in acidic medium and disproportionates. ltBrgt 3MnO2−4+4H+→MnO2+2MnO−4+2H2O
3MnO2−4+2H2O→2MnO−4+MnO2+4OH−
Topper's Solved these Questions
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Exercise 2
40 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Exercise 3
65 Videos
- D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS EXERCISE - Match the column
1 Video
- CHEMICAL EQUILIBRIUMBOOK - RESONANCE ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - Advanced Level Problems (Part-3)(Stage-5)
2 Videos
- GASEOUS STATEBOOK - RESONANCE ENGLISHCHAPTER - GASEOUS STATEEXERCISE - ORGANIC CHEMISTRY(Hydrocarbon)
17 Videos
Similar Questions
Match the compounds of column X with oxidation state of central atom in column Y.
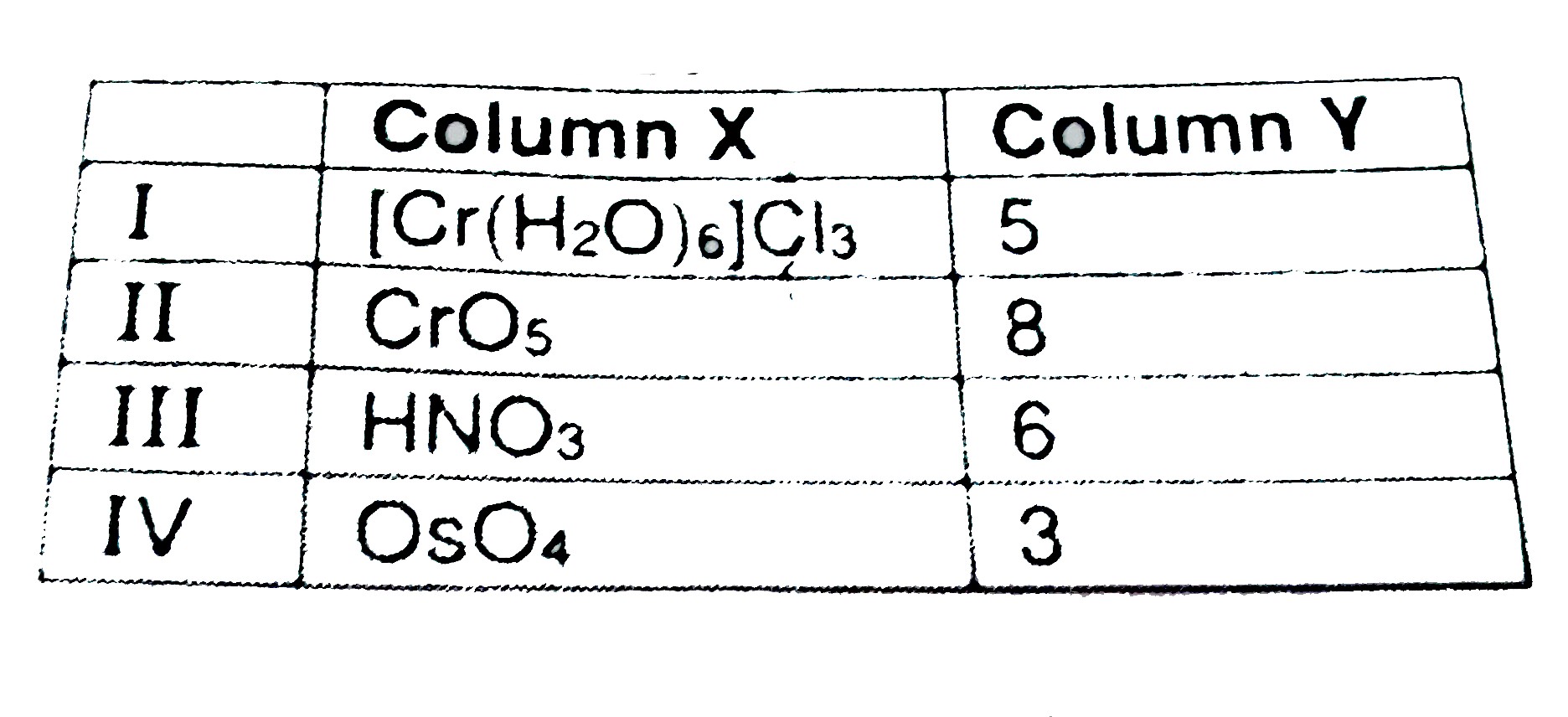 .
.
View SolutionIdentify the species in which the metal atom is in +6 oxidation state.
View SolutionCuSO4 reacts with NH3 to give blue complex of
View SolutionAn alloy consists of rubidium and one of the other alkali metals. A sample of 4.6g of the alloy when allowed to react with water, liberates 2.241dm3 of hydrogen at STP.
Relative atomic masses: AT(Li)=7,AT(Na)=23,AT(K)=39,AT(Rb)=85.5,AT(Cs)=1.33
Which alkali metal is the component of the alloy?
View SolutionA certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
View SolutionWhich type of reaction MnO2−4show with acid , dilute -alkali or water.
View SolutionWhich of the following are exothermic processes? 1. Reaction of water with quick lime 2. Dilution of an acid 3. Evaporation of water 4. Sublimation of camphor (crystals)
View SolutionMnO_(4)^(2-) undergoes disproportionation reaction in acidic medium but MnO_(4)^(-) does not. Given reason.
View SolutionWhich type of reaction MnO2−4 shows with aid, dilute-alkali or water.
View SolutionIn acid solution, the reaction MnO_(4)^(-) rarr Mn^(2+) involves
View Solutionदानेदार जिंक की तनु, अम्ल अथवा क्षार से अभिक्रिया द्वारा गैस प्राप्त होती है। (हाइड्रोजन/नाइट्रोजन)
View SolutionMnO2−4 अम्लीय माध्यम में असमानुपातं अभिक्रिया प्रदर्शित करता है परन्तु MnO−4 नहीं करता है | कारण भी दीजिए |
View Solutionदानेदार जिंक की तनु, अम्ल अथवा क्षार से अभिक्रिया द्वारा गैस प्राप्त होती है। (हाइड्रोजन/नाइट्रोजन)
View SolutionIn acidic medium, reaction MnO_(4)^(-) hArr Mn^(2+) is an example of -
View SolutionWhich sulphide is insoluble in dilute acids but soluble in alkalies:
View SolutionMnO_(4)^(2-) undergoes disproportionation reaction in acidic medium but MnO_(4)^(-) does not .Give reason.
View Solution

