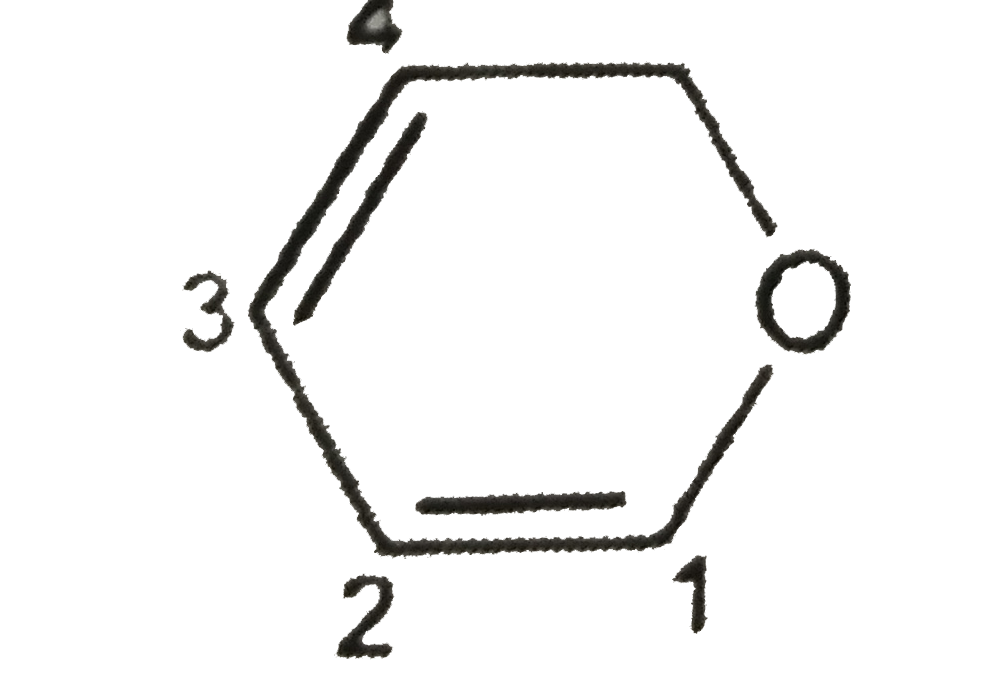
In this molecules, π-electron density is more on :
C1andC3
C2andC4
C2andC3
C1andC4
The correct Answer is:B
Step by step video solution for In this molecules, pi-electron density is more on : by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:EXERCISE-2 PART-II SINGLE AND DOUBLE VALUE INTEGER TYPE
Explore 11 Videos
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:EXERCISE-2 PART-III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE
Explore 9 Videos
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:EXERCISE-1 PART-III (MATCH THE COLUMN)
Explore 1 Video
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Part-III: Section-5: Matching List Type
Explore 1 Video
- HYDROGEN AND ITS COMPOUNDSBook:RESONANCE ENGLISHChapter:HYDROGEN AND ITS COMPOUNDSExercise:INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)
Explore 33 Videos
Similar Questions
Knowledge Check
In which of the following molecules, π− electron density in ring is minimum ?
A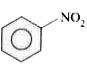 B
B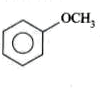 C
C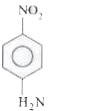 D
D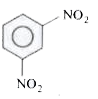
In which of the following molecules π−electron density in ring is maximum?
A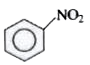 B
B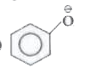 C
C D
D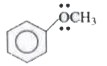
The resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacentatom.' The effect is transmitted through the chain.
In positive resonance effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. In negative resonance effect, the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
Which of the following does not show resonance effect ?
View SolutionThe resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacentatom.' The effect is transmitted through the chain.
In positive resonance effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. In negative resonance effect, the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
Which of the following shows +M effect ?
View SolutionThe resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacentatom.' The effect is transmitted through the chain.
In positive resonance effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. In negative resonance effect, the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
Which of the following carboxylate ions is the most stable ?
View SolutionThe resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacentatom.' The effect is transmitted through the chain.
In positive resonance effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. In negative resonance effect, the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
What is the IUPAC name for :
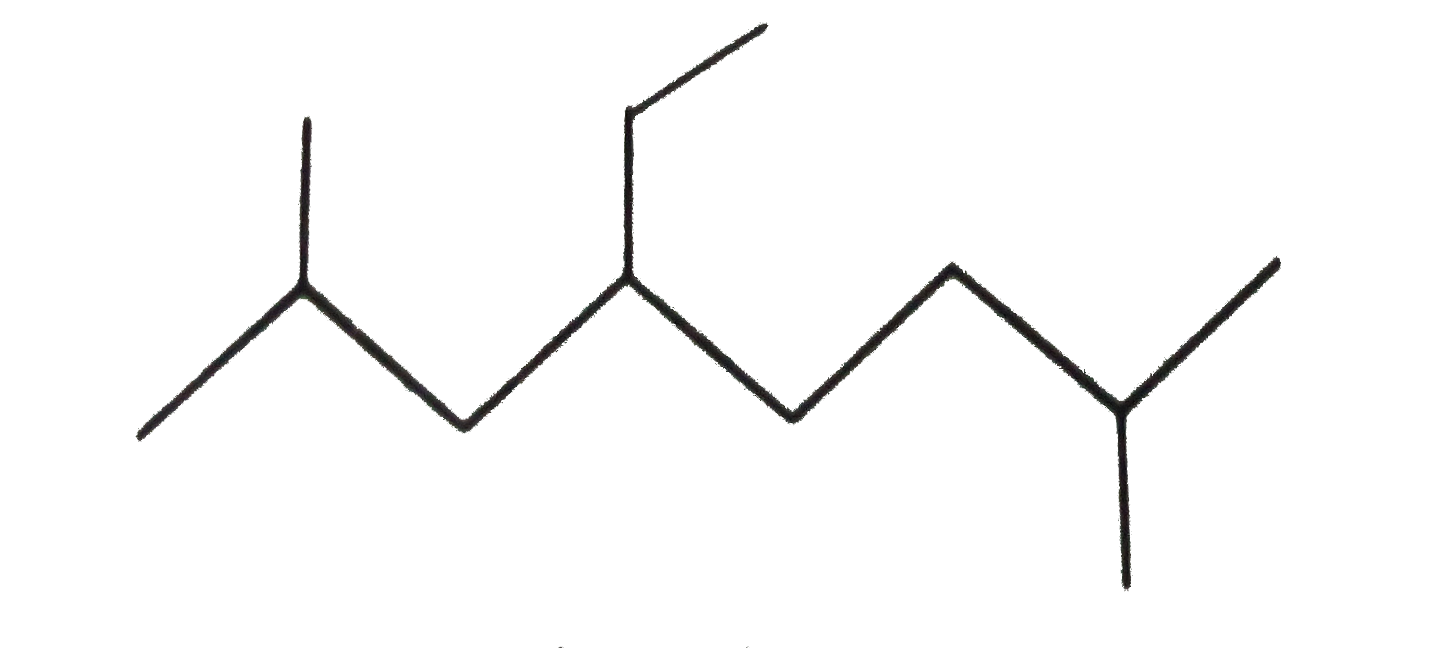
View SolutionThe resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacentatom.' The effect is transmitted through the chain.
In positive resonance effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. In negative resonance effect, the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
Which of the following intermediates is pyramidal in shape ?
View SolutionThe resonance effect is defined as 'the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacent atom.' The effect is transmitted through the chain.
In positive resonance effect, the transfer of electrons is away from an atom or substituent group attached to the conjugated system. This electron displacement makes certain positions in the molecule of high electron densities. In negative resonance effect, the transfer of electrons is towards the atom or substituent group attached to the conjugated system.
Which of the following free radicals is the most stable ?
View Solution
