Which of the following resonating structures of 1-methoxy-1,3-butadiene is least stable ?
A
ΘCH2−CH=CH−CH=⊕O−CH3
B
CH2=CH−ΘCH−CH=⊕O−CH3
C
CH2=CH−⊕CH−ChΘCH−O−CH3
D
CH2=CH−ΘCH−⊕CH−O−CH3
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video solution for Which of the following resonating structures of 1-methoxy-1,3-butadiene is least stable ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:PART-III : PRACTICE TEST-5
Explore 1 Video
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:PART-III : PRACTICE TEST-6
Explore 1 Video
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:PART-III : PRACTICE TEST-3
Explore 1 Video
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Part-III: Section-5: Matching List Type
Explore 1 Video
- HYDROGEN AND ITS COMPOUNDSBook:RESONANCE ENGLISHChapter:HYDROGEN AND ITS COMPOUNDSExercise:INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)
Explore 33 Videos
Similar Questions
Knowledge Check
Which of the following represents resonating structure of
 A
A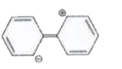 B
B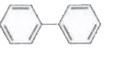 C
C DAll of these
DAll of theseWhich of the following is not resonating structure of each other?
ACH3−N=C=SandCH3−S−C≡NBCH3−+C=OandCH3−C≡+OCCH3−O∣∣C−OHandCH3−O−∣C=+O−HDCH2=CH−C≡Nand+CH2−CH=C=N−Which of the following is pair of resonance structure?
A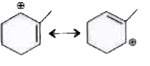 B
B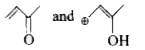 C
C D
D
Which of the following is least stable?
View SolutionWhich of the following is not a resonance structure of the other?
View SolutionWhich of the following resonating structure is the least contributing structure ?
View SolutionWhich of the following is least stable ?
View SolutionWhich of the following is least stable ?
View Solution
