In the free-radical chlorination of methane, the chain-intiation step involves the formation of:
Chlorine radical
Hydrogen chloride
Methyl radical
Chloromethyl radical
The correct Answer is:A
Step by step video solution for In the free-radical chlorination of methane, the chain-intiation step involves the formation of: by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - Exercise-1 Part-3
2 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - Exercise-2 Part-1
11 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 3
22 Videos
- MOLE CONCEPTBOOK - RESONANCE ENGLISHCHAPTER - MOLE CONCEPTEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
27 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - APSP PART-3
22 Videos
Similar Questions
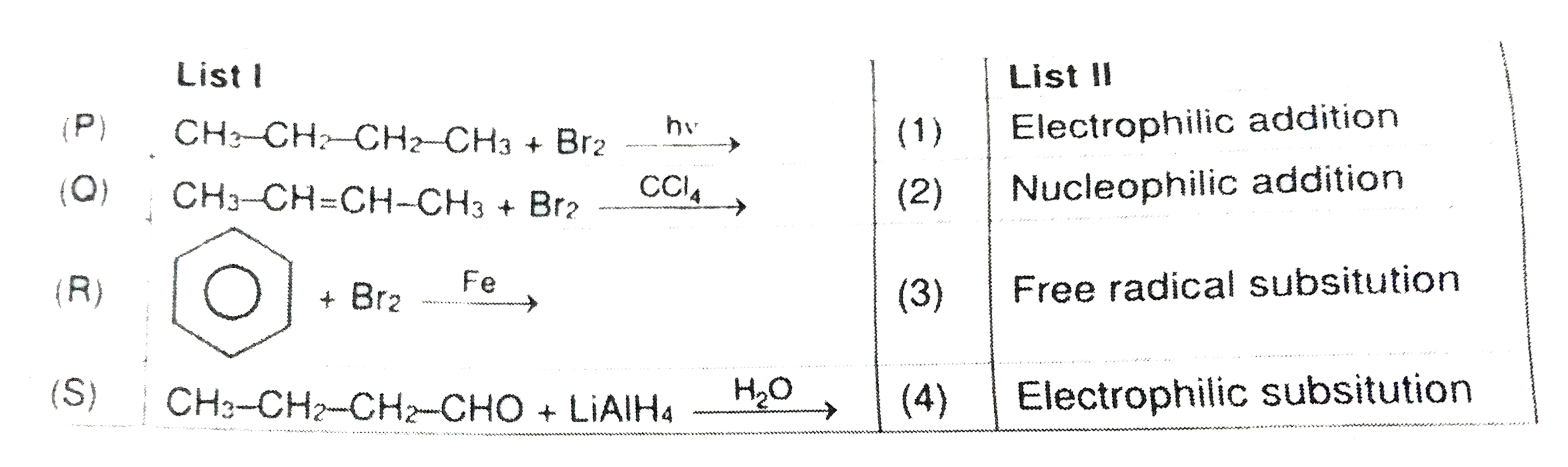
Match List I (Reaction ) with List II (Type of reaction ) and select the correct answer using the code given below the lists :
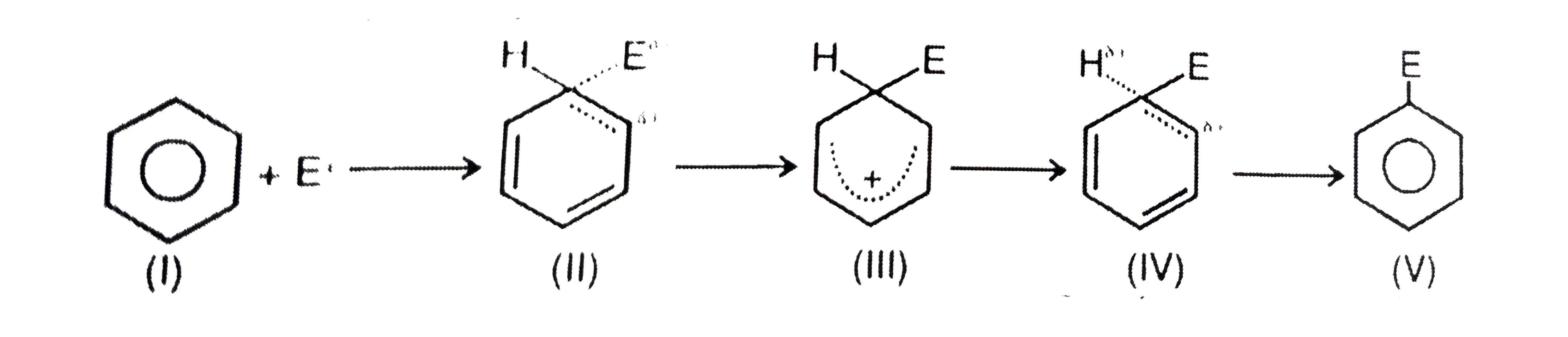
View SolutionWhich of the following species is expected to have maximum enthalpy in an electrophilic aromatic substitution reaction ?
View Solution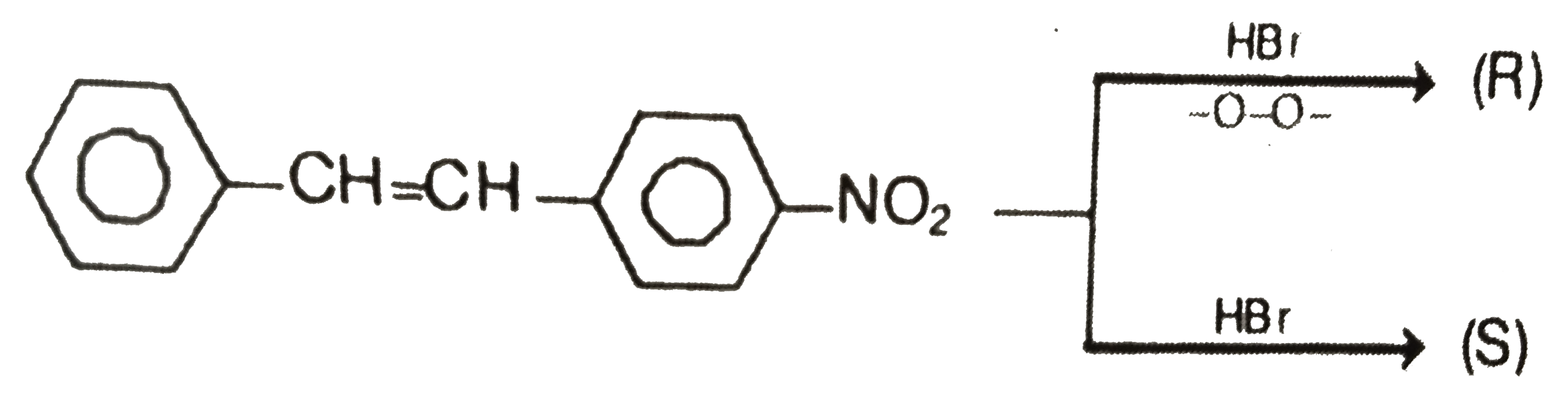
R & S are :
View SolutionA partially dried clay mineral contains 8%d water. The original sample contained 12% water and 45% sillica.
The % if sillica in the partially dried sample is nearly:
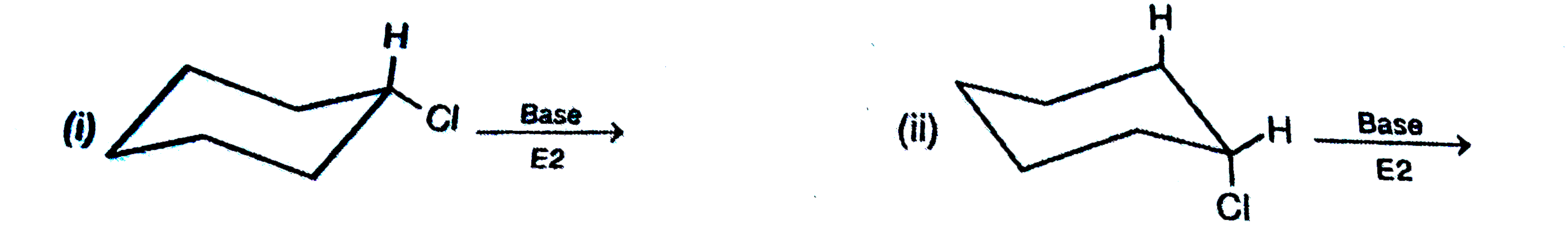
View SolutionRate of Bimolecular elimination (E2) reaction for the following :
View SolutionIn the free-radical chlorination of methane, the chain-intiation step involves the formation of:
View SolutionAssertion : In vulcanisation of rubber , sulphur cross links are introduced. Reason : Vulcanisation is a free radical intiated chain reaction.
View SolutionHunsdiecker reaction involve free radical in intermediate steps.
View SolutionIn the free radical chlorination of methane, the chain initiating step involves the formation of
View SolutionIn the chlorination of Methane which of the following reaction involve in the chain termination step .
View SolutionThe photochemical chlorination of paraffins occurs by a free radical mechanism. From the following set of reactions pick out the chain propogation steps.
View SolutionIn the chlorination of alkanes, the first step in which chlorine free radicals are produced is called
View SolutionChlorine free radicals react with methane by
View SolutionArrange the steps according to the order followed in the free radical polymerisation.
(i) Chain initiating step
(ii) Chain terminating step
(iii) Free radical formation
(iv) Chain propagation step
View Solutionमेथेन के मुक्त मूलक क्लोरोनीकरण में श्रृखंला के आरम्भिक पद में निम्नलिखित में से किसकी उत्पत्ति होती है?
View SolutionWhich of the following steps represents chain terminating step in the mechanism of chlorination of methane ?
View Solution

