In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti−Markovnikov's addition to alkenes because :
both are highly ionic
one is oxidising and the other is reducing
one of the steps is endothermic in both the cases
all the steps are exothermic in both the cases.
The correct Answer is:C
Step by step video solution for In the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov's addition to alkenes because : by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - Exercise-3 Part-2
34 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 1
30 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - Exercise-2 Part-4
5 Videos
- MOLE CONCEPTBOOK - RESONANCE ENGLISHCHAPTER - MOLE CONCEPTEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
27 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - APSP PART-3
22 Videos
Similar Questions
What is the product when acetylene reacts with HOCl
View Solution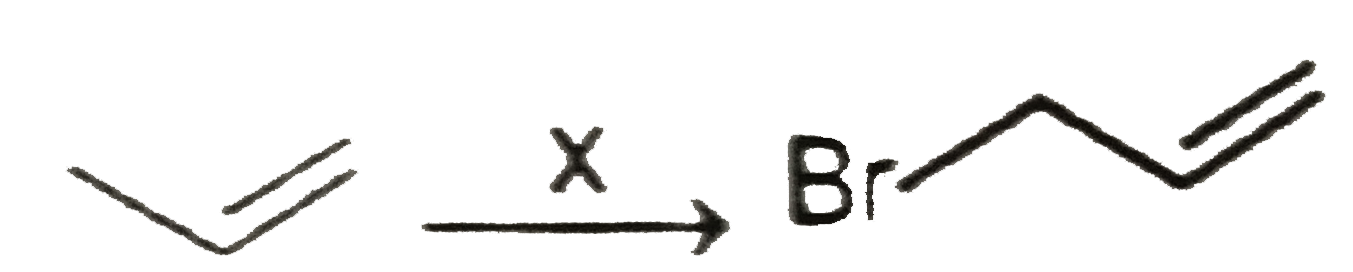
'X' is
View SolutionThree acyclic alkenes (x,y,z) on catalytic hyrogenation give same alkane. On reaction with HCl (x,y,z) form same major tertiary halide product.Reductive ozonolysis of mixture of (x,y,z) gives a mixture of two moles of CH2=O one moles of CH3CH=O one mole of acetone, one mole of butanone and one mole of 2-methyl propanal, x,y and z do not have any stereoisomers.
(x,y,z)H3O∗−−−→ addition product . The correct statement is
View SolutionA partially dried clay mineral contains 8%d water. The original sample contained 12% water and 45% sillica.
The % if sillica in the partially dried sample is nearly:
View SolutionRate of Bimolecular elimination (E2) reaction for the following :
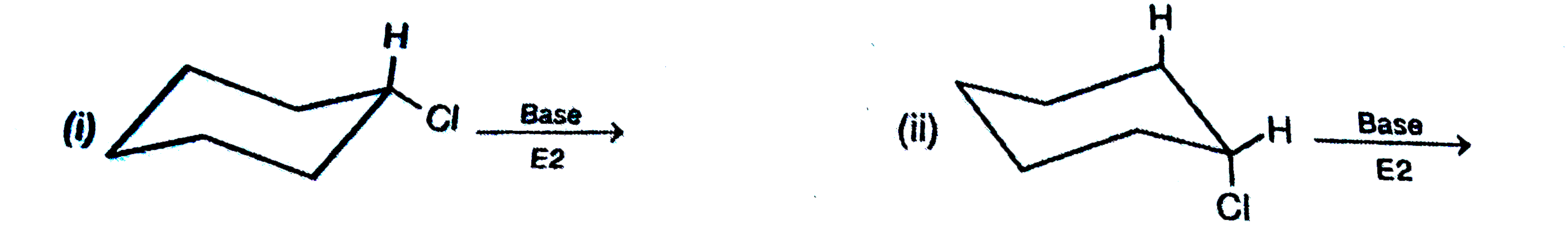
View SolutionThe anti-Markovnikov's addition of HBr to alkenes in the presence of peroxides was rationalized by
View SolutionIn the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov's addition to alkenes because:
View SolutionIn the presence of peroxide, hydrogen chloride and hydrogen iodide do not give Anti Markownikov's addition to alkenes because
View SolutionIn the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov addition to alkenes because :
View SolutionIn the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti Markownikoff's addition to alkenes because
View SolutionIn presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov's addition to alkenes because
View SolutionIn the presence of peroxide HCl do not give anti Markownikoffs addition to alkene because
View SolutionIn the presence of peroxide, hydrogen chloride and hydrogen iodide do not give anti-Markovnikov's addiition to alkenes because
View Solutionपरॉक्साइड की उपस्थिति में हाइड्रोजन क्लोराइड तथा हाइड्रोजन आयोडाइड एल्कीन के साथ एन्टी-मार्कोनीकॉफ योग नहीं देते क्योंकि
View SolutionThe addition of HCl in presence of peroxide does not follow anti-Markovnikov's rule because
View Solutionपरॉक्साइड की उपस्थिति में हाइड्रोजन क्लोराइड तथा हाइड्रोजन आयोडाइड एल्कीन के साथ एन्टी-मार्कोनीकॉफ योग नहीं देते क्योंकि
View Solution

