Which of the following statements is correct ?
−NO2 group activates the benzene ring for attack of electrophile at ortho and para position
−NH2 group activates the benzene ring for attack of electrophile at ortho and para position
Both −NO2 group as well as −NH2 group activate the benene ring for attack of electrophile at ortho and para position.
Neither −NO2 group nor −NH2 group activate the benzene ring the attack of electrophile at ortho and para position.
The correct Answer is:B
To determine which statement is correct regarding the activation of the benzene ring for electrophilic substitution reactions by the groups NO2 and NH2, we can analyze the effects of these substituents on the aromatic ring.
1. Understanding Electrophilic Substitution:
- Electrophilic aromatic substitution is a reaction where an electrophile replaces a hydrogen atom on the benzene ring. The position where the electrophile attacks the ring can be influenced by the substituents already present on the ring.
2. Effect of NO2 Group:
- The nitro group (NO2) is a strong electron-withdrawing group due to its resonance and inductive effects. It pulls electron density away from the benzene ring.
- This electron-withdrawing effect makes the benzene ring less reactive towards electrophiles, and it directs incoming electrophiles to the meta position rather than ortho or para positions.
3. Effect of NH2 Group:
- The amino group (NH2) is an electron-donating group. It donates electron density to the benzene ring through resonance, increasing the electron density at the ortho and para positions.
- This makes the benzene ring more reactive towards electrophiles and directs incoming electrophiles to the ortho and para positions.
4. Analyzing the Statements:
- Statement 1: "NO2 group activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is incorrect because the NO2 group deactivates the ring and directs electrophiles to the meta position.
- Statement 2: "NH2 group activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is correct because the NH2 group increases the reactivity of the benzene ring and directs electrophiles to the ortho and para positions.
- Statement 3: "Both NO2 as well as NH2 activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is incorrect because while NH2 activates the ring, NO2 does not.
- Statement 4: "Neither NO2 nor NH2 group activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is incorrect because NH2 does activate the ring.
5. Conclusion:
- The correct statement is: "NH2 group activates the benzene ring for the attack of electrophile at ortho and para position."
Final Answer:
The correct statement is: NH2 group activates the benzene ring for the attack of electrophile at ortho and para position.
- Electrophilic aromatic substitution is a reaction where an electrophile replaces a hydrogen atom on the benzene ring. The position where the electrophile attacks the ring can be influenced by the substituents already present on the ring.
- The nitro group (NO2) is a strong electron-withdrawing group due to its resonance and inductive effects. It pulls electron density away from the benzene ring.
- This electron-withdrawing effect makes the benzene ring less reactive towards electrophiles, and it directs incoming electrophiles to the meta position rather than ortho or para positions.
- The amino group (NH2) is an electron-donating group. It donates electron density to the benzene ring through resonance, increasing the electron density at the ortho and para positions.
- This makes the benzene ring more reactive towards electrophiles and directs incoming electrophiles to the ortho and para positions.
- Statement 1: "NO2 group activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is incorrect because the NO2 group deactivates the ring and directs electrophiles to the meta position.
- Statement 2: "NH2 group activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is correct because the NH2 group increases the reactivity of the benzene ring and directs electrophiles to the ortho and para positions.
- Statement 3: "Both NO2 as well as NH2 activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is incorrect because while NH2 activates the ring, NO2 does not.
- Statement 4: "Neither NO2 nor NH2 group activates the benzene ring for the attack of electrophile at ortho and para position."
- This statement is incorrect because NH2 does activate the ring.
- The correct statement is: "NH2 group activates the benzene ring for the attack of electrophile at ortho and para position."
The correct statement is: NH2 group activates the benzene ring for the attack of electrophile at ortho and para position.
Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 3
22 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 1
30 Videos
- MOLE CONCEPTBOOK - RESONANCE ENGLISHCHAPTER - MOLE CONCEPTEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
27 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - APSP PART-3
22 Videos
Similar Questions
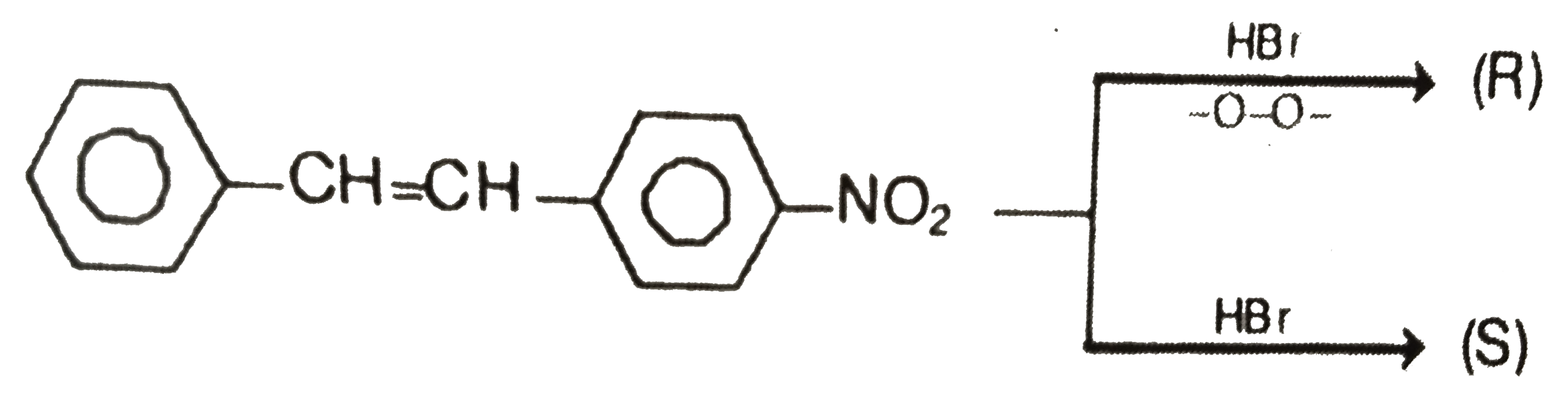
R & S are :
View Solution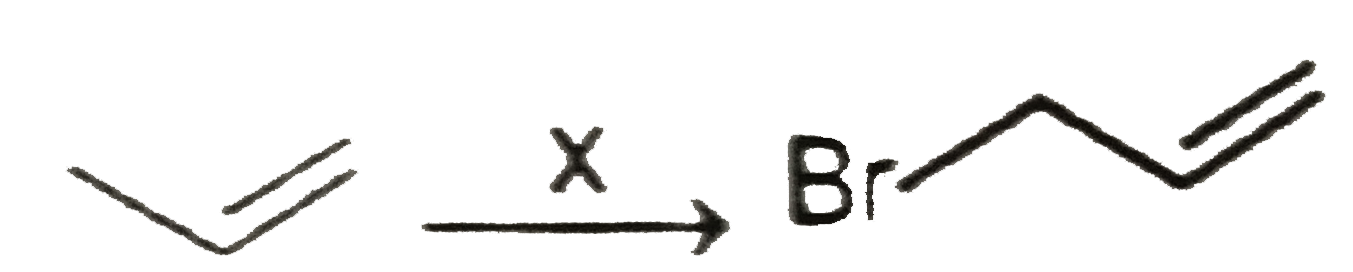
'X' is
View SolutionA partially dried clay mineral contains 8%d water. The original sample contained 12% water and 45% sillica.
The % if sillica in the partially dried sample is nearly:
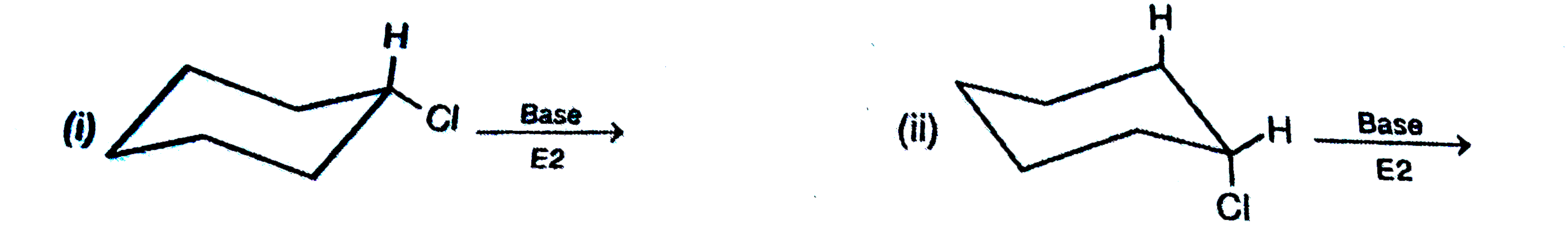
View SolutionRate of Bimolecular elimination (E2) reaction for the following :
View SolutionWhich of the following statements are correct statements ?
View SolutionWhich of the following statement is correct
View SolutionWhich of the following statements is/are correct?
View SolutionWhich of the following statements is not correct ?
View SolutionWhich of the following is/are correct statement/statements ?
View SolutionWhich of the following statement is not correct ?
View SolutionWhich of the following statements is correct ?
View SolutionWhich of the following statement is/are correct ?
View SolutionWhich of the following statement is correct ?
View SolutionWhich of the following statements is correct?
View SolutionWhich of the following statements are correct ?
View SolutionWhich of the following statements is (are) correct ?
View Solution

