Alcohols undergo acid catalysed elimination reactions to produce alkenes. Because water is lost in the elimination , this reaction is called dehydration reaction. Secondary and tertiary alcohols always give E1 reaction in dehydration. Primary alcohols whose β-carbon is branched also give E1 reaction. The reactivity of alcohol for elimination reaction is tertiary alcohol > secondary alcohol > Primary alcohol.
Which of the following dehydration product (major ) is incorrect ?
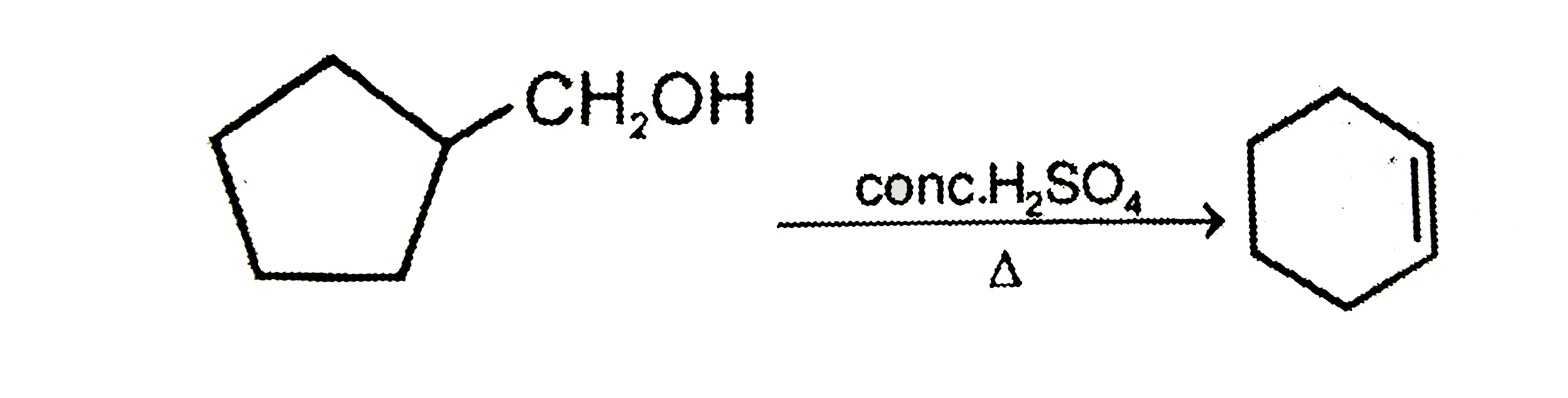
CH3−CH3∣C∣CH3−OH∣CH−CH3conc.H2SO4−−−−−−−→ΔCH3−CH3∣C∣CH3−CH=CH2
CH3CH2CH2CH2OHconc.H2SO4−−−−−−−→ΔCH3−CH=CH−CH3
The correct Answer is:B
To solve the question regarding the dehydration products of alcohols, we will analyze the dehydration reactions for each of the provided alcohols and identify which major product is incorrectly stated.
1. Understanding the Mechanism:
- Alcohols undergo acid-catalyzed dehydration reactions to form alkenes. This process involves the formation of a carbocation intermediate and can follow either E1 or E2 mechanisms.
- Secondary and tertiary alcohols typically undergo E1 reactions, while primary alcohols with branched beta-carbons can also undergo E1 reactions.
2. Analyzing Each Alcohol:
- Example 1: A primary alcohol with a branched beta-carbon.
- Upon protonation, the alcohol forms a carbocation. If a ring expansion occurs, it leads to a more stable carbocation, which then loses a proton to form an alkene. This product is correct.
- Example 2: A tertiary alcohol.
- The tertiary alcohol undergoes protonation, leading to a stable tertiary carbocation. A methyl shift occurs, resulting in a more stable carbocation. The major product formed here is the more stable alkene. However, if the stated major product is not the one derived from the most stable carbocation, it is incorrect.
- Example 3: A primary alcohol (butanol).
- The butanol undergoes dehydration to form a primary carbocation, which rearranges to a more stable secondary carbocation via a hydride shift. The major product here is 2-butene, which is correct.
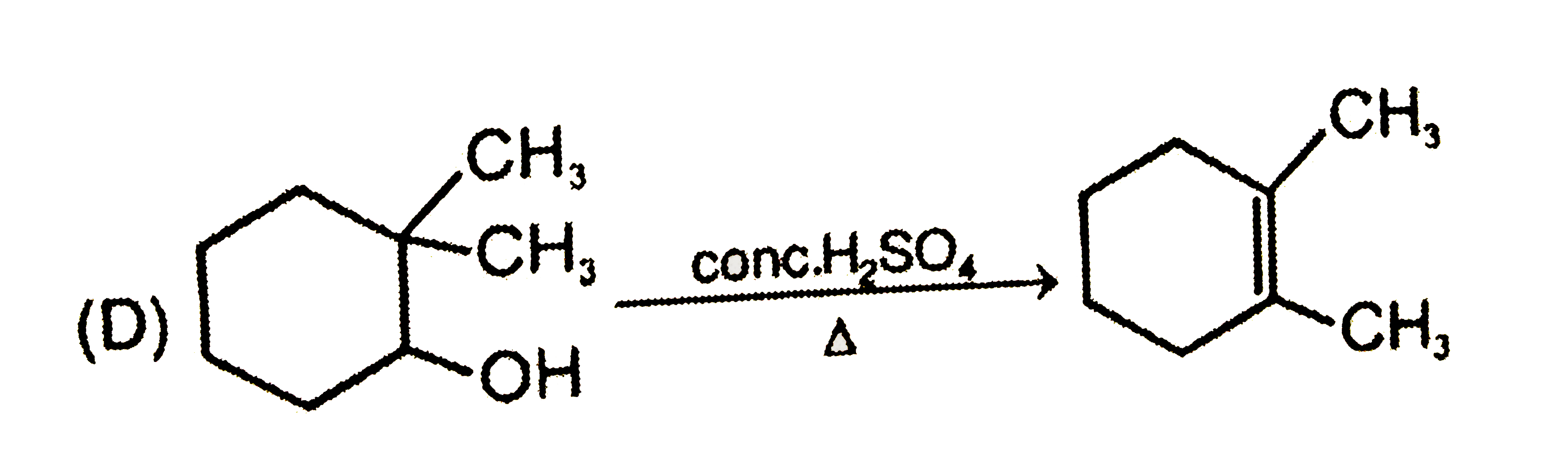
- Example 4: Another tertiary alcohol.
- Similar to Example 2, this alcohol undergoes protonation to form a tertiary carbocation. The product formed after losing a proton is the expected alkene. If the stated product does not match the expected alkene, it is incorrect.
3. Identifying the Incorrect Product:
- After analyzing all examples, we find that the major product from the tertiary alcohol in Example 2 is incorrectly stated. The correct major product should be the one derived from the most stable carbocation formed after rearrangement.
Conclusion:
The incorrect major product is from the second example, where the expected major product does not align with the correct product formed through the dehydration mechanism.
- Alcohols undergo acid-catalyzed dehydration reactions to form alkenes. This process involves the formation of a carbocation intermediate and can follow either E1 or E2 mechanisms.
- Secondary and tertiary alcohols typically undergo E1 reactions, while primary alcohols with branched beta-carbons can also undergo E1 reactions.
- Example 1: A primary alcohol with a branched beta-carbon.
- Upon protonation, the alcohol forms a carbocation. If a ring expansion occurs, it leads to a more stable carbocation, which then loses a proton to form an alkene. This product is correct.
- Example 2: A tertiary alcohol.
- The tertiary alcohol undergoes protonation, leading to a stable tertiary carbocation. A methyl shift occurs, resulting in a more stable carbocation. The major product formed here is the more stable alkene. However, if the stated major product is not the one derived from the most stable carbocation, it is incorrect.
- The butanol undergoes dehydration to form a primary carbocation, which rearranges to a more stable secondary carbocation via a hydride shift. The major product here is 2-butene, which is correct.
- Similar to Example 2, this alcohol undergoes protonation to form a tertiary carbocation. The product formed after losing a proton is the expected alkene. If the stated product does not match the expected alkene, it is incorrect.
- After analyzing all examples, we find that the major product from the tertiary alcohol in Example 2 is incorrectly stated. The correct major product should be the one derived from the most stable carbocation formed after rearrangement.
The incorrect major product is from the second example, where the expected major product does not align with the correct product formed through the dehydration mechanism.
টপার এই প্রশ্নগুলো সমাধান করেছে
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-3 Part-1
18 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-3 Part-2 JEE (MAIN ) OFFLINE PROBLEMS
10 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-2 Part-3
10 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 3
22 Videos
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
24 Videos
সম্পর্কিত প্রশ্ন
Alcohols undergo acid catalysed elimination reactions to produce alkenes. Because water is lost in the elimination , this reaction is called dehydration reaction. Secondary and tertiary alcohols always give E1 reaction in dehydration. Primary alcohols whose β-carbon is branched also give E1 reaction. The reactivity of alcohol for elimination reaction is tertiary alcohol gt secondary school gt Primary alcohol.
Identify the product in the given reaction :
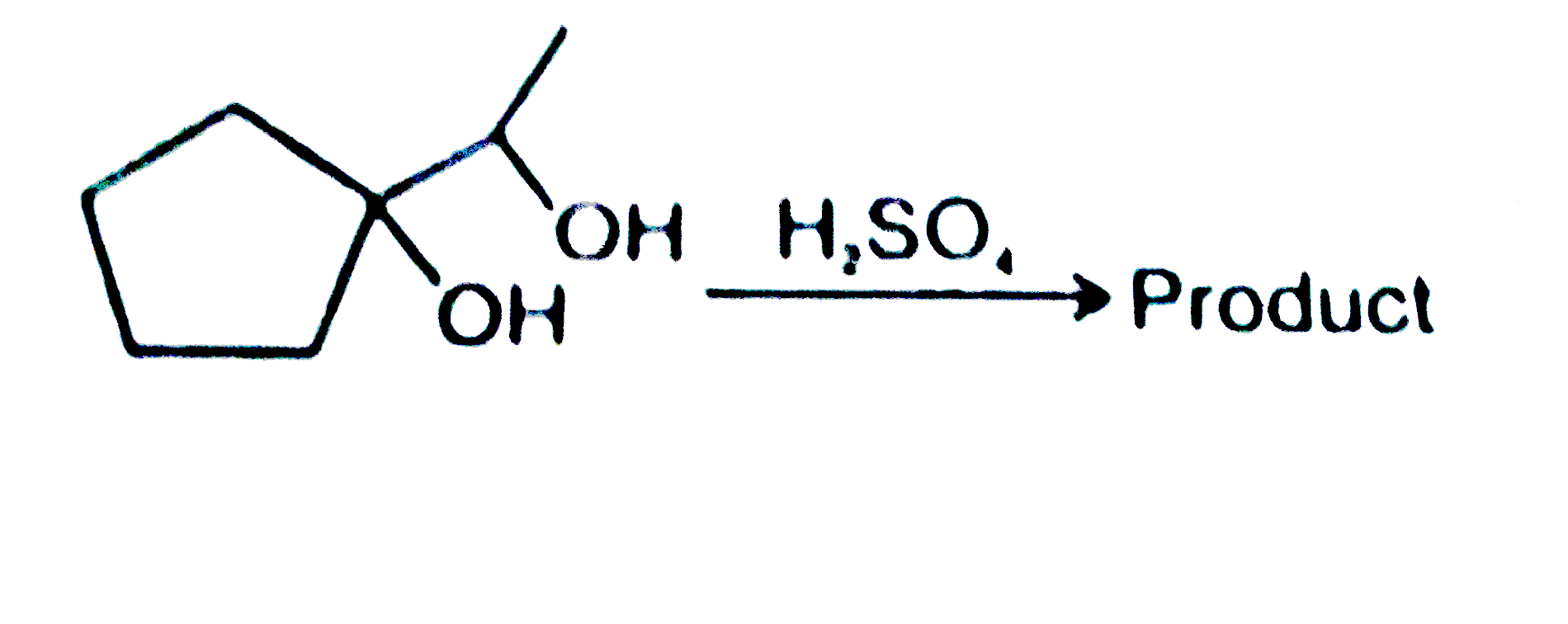
View SolutionIs isobutyl alcohol a secondary alcohol ?
View Solution
জ্ঞান পরীক্ষা করুন
In esterfication reaction rate for different alcohols
A3∘>1∘>2∘B2∘>3∘>1∘C1∘>2∘>3∘Dnone of these
What is the order of dehydration of primary, secondary and tertiary alcohols?
View SolutionIn the esterification reaction of alcohols
View SolutionAlcohol can be prepared from alken by which of the following reaction
View SolutionPrimary , secondary and tertiary alcohols can be distinguished hy
View SolutionWhich one of the following reaction would produce secondary alcohol?
View SolutionWhich of the following reaction leads to the formation of secondary alcohol?
View SolutionWhich of the following on reaction with conc. NaOH gives an alcohol ?
View Solution

