The major product of the following reaction is :
CH3CH2C∣BrH−C∣BrH2I.KOHalc.−−−−−−−−−−−−→II.NaNH2in liq.NH3
CH3CH=C=CH2
CH3CH=CHCH2NH2
CH3CH2C≡CH
CH3CH2C∣NH2H−C∣NH2H2
The correct Answer is:C
Step by step video solution for The major product of the following reaction is : CH_(3)CH_(2)underset(Br)underset(|)CH-underset(Br)underset(|)CH_(2) underset(II. NaNH_(2) "in liq." NH_(3))overset(I.KOH alc.)to by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - APSP PART-1
29 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - APSP PART-2
17 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - Exercise-3 Part-2 JEE (MAIN ) OFFLINE PROBLEMS
10 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 3
22 Videos
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
24 Videos
Similar Questions
CH3−C∣∣O−CH3+PhMgBr→AH3O+−−−→BH3PO4−−−→ΔC
C is :
View SolutionAn alkyl bromide produces a single alkene when it reacts with sodium ethoxide and ethanol. This alkene undergoes hydrogenation and produces 2-methylbutane. What is the alkybromide ?
View SolutionMaximum dehydration takes place that of-
View Solution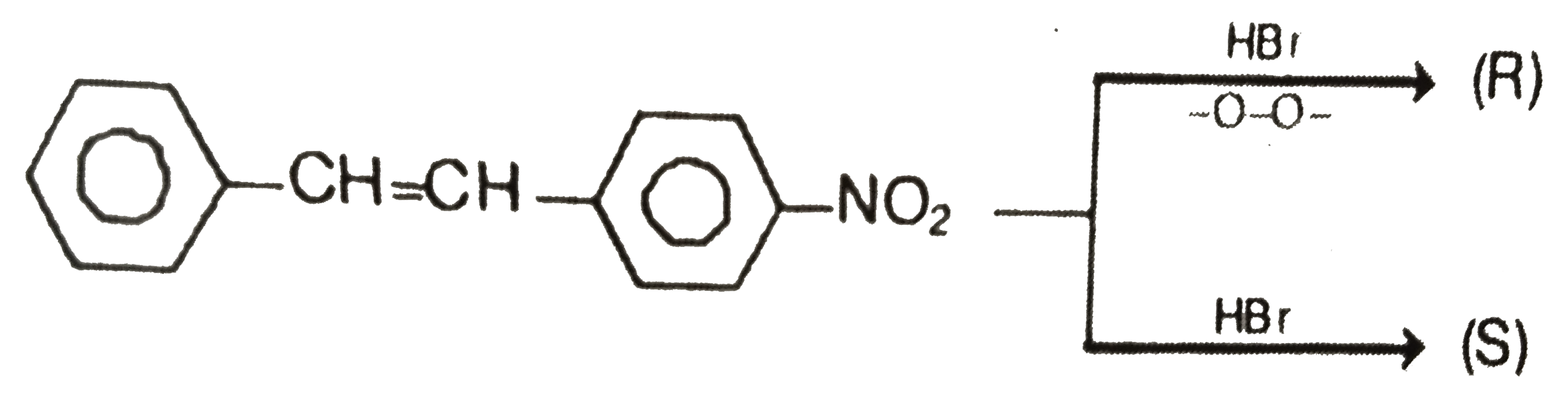
R & S are :
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionConsider the following reactions: underset(Br)underset(|)(CH_(3))-underset(Br)underset(|)(CH_(2)) underset(H_(2)O,Delta)overset(NaOH)toA overset(NaH)toB,B+underset(Br)underset(|)(CH_(2))-underset(Br)underset(|)(CH_(2))toC The major product formed is:
View SolutionCH_(3)-underset((a))underset(Br)underset(|)overset(CH_(3))overset(|)C-CH_(3)," "CH_(3)-underset((b))underset(Br)underset(|)CH-CH_(3)," "CH_(3)-underset((C))CH-Br Compare rate of E_(2) reaction :
View SolutionIn the reaction
CH3CH2C∣BrHCH2CH=CH2KOH(alc.)−−−−−→A
The product A is predominantly.
View SolutionCH2∣Br−CH=CH−CH2∣BrZnCH3OH−−−−−−→ Product The predominating product is
View Solutionनिम्नलिखित यौगिकों के IUPAC नाम लिखिए-
(a) CH3−Br∣CH−Br∣CH−COOC2H5
(b) (CH3)3C.CH2.CH2Cl
(c) CH3−CH2−CH∣Br−CH∣CH3−CH3∣C∣CH3−CH3
(d) CH3−CH2−CH∣Br−CH3∣C∣CH3−CH2Cl
View SolutionThe major product of the following reaction is : {:(CH_(3)CH_(2)CH-CH_(2) ),(" |"" |"),(" "Br" " Br):} underset(underset("in liq" NH_(3))(II. NaNH_(2)))overset("I. KOH alc")rarr
View SolutionIn the reaction
H−C≡CH(1)NaNH2/liq.NH3−−−−−−−−−−−−−−→(2)CH3CH2BrX(1)NaNH2/liq.NH3−−−−−−−−−−−−−−→(2)CH3CH2BrY
X and Y are -
View SolutionThe major product of the following reaction is :
CH3CH2C∣BrH−C∣BrH2I.KOHalc.−−−−−−−−−−−−→II.NaNH2in liq.NH3
View SolutionCH_(3)-CH_(2)-underset(Br)underset(|)(CH)-CH_(3)underset(Delta)overset("alc.KOH")rarrX (Major)
View Solutionअभिक्रिया में
H−C≡CH(i)NaNH2/liq.NH3−−−−−−−−−−−−−→(ii)CH3CH2Br
X(i)NaNH2/Hq.NH3−−−−−−−−−−−−−→(ii)CH3CH2BrY.
X और Y है
View SolutionCH_(3)-CH= CH-CH_(3)+ Br_(2) to CH_(3)-underset(Br)underset(|)CH-underset(Br)underset(|)CH-CH_(3) is an
View Solution

