What product is obtained when Benzencarbonitrile is hydrolysed.
Benzoylchloride
Benzenecarboxamide
Benzaldehyde
Benzoic acid
The correct Answer is:D
To determine the product obtained when benzene carbonitrile is hydrolyzed, we can follow these steps:
Step 1: Identify the structure of benzene carbonitrile
Benzene carbonitrile, also known as benzonitrile, has the chemical formula C6H5CN. It consists of a benzene ring (C6H5) attached to a nitrile group (CN).
Step 2: Understand hydrolysis
Hydrolysis is a chemical reaction involving water that leads to the breakdown of a compound. In the case of nitriles, hydrolysis typically converts the nitrile group into a carboxylic acid.
Step 3: Write the hydrolysis reaction
When benzene carbonitrile undergoes hydrolysis, it reacts with water (H2O). The reaction can be represented as follows:
C6H5CN+H2O→C6H5COOH+NH3
Step 4: Identify the product
The product of this hydrolysis reaction is benzoic acid (C6H5COOH) and ammonia (NH3).
Conclusion
Thus, when benzene carbonitrile is hydrolyzed, the main product obtained is benzoic acid.
Final Answer
The product obtained when benzene carbonitrile is hydrolyzed is benzoic acid.
---
Benzene carbonitrile, also known as benzonitrile, has the chemical formula C6H5CN. It consists of a benzene ring (C6H5) attached to a nitrile group (CN).
Hydrolysis is a chemical reaction involving water that leads to the breakdown of a compound. In the case of nitriles, hydrolysis typically converts the nitrile group into a carboxylic acid.
When benzene carbonitrile undergoes hydrolysis, it reacts with water (H2O). The reaction can be represented as follows:
The product of this hydrolysis reaction is benzoic acid (C6H5COOH) and ammonia (NH3).
Thus, when benzene carbonitrile is hydrolyzed, the main product obtained is benzoic acid.
The product obtained when benzene carbonitrile is hydrolyzed is benzoic acid.
टॉपर्स ने हल किए ये सवाल
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-III Match the column
Explore 2 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Exercise-2 (Part-I: Only One option correct Type)
Explore 10 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Exercise 1 (Part-I Subjective Questions)
Explore 19 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 24 Videos
- S BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:S BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 4 Videos
समान प्रश्न
ज्ञान जांच
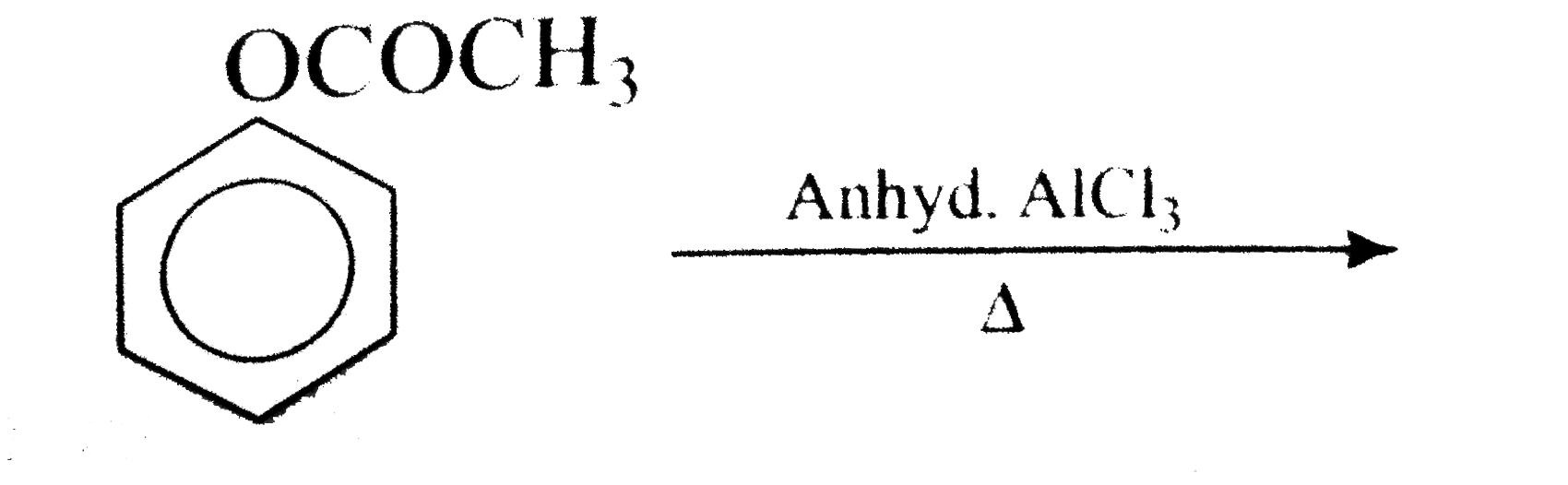
 , Products obtained are : ARacenicBDiastereomersCG.IDPositional isomers
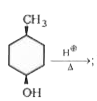
, Products obtained are : ARacenicBDiastereomersCG.IDPositional isomers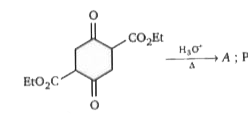 , Product obtained is :A
, Product obtained is :A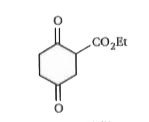 B
B C
C DNone of these
DNone of these
What product is obtained when ethylbenzene is oxidised with alkaline KMnO4?
View SolutionWhat product is obtained when ethylbenzene is oxidised with alkaline KMnO4?
View SolutionName the products obtained when zinc blende is roasted .
View SolutionWhich of the following characteristics of products obtained when green vitriol is strongly heated?
View SolutionName the products obtained when limestone is calcined.
View SolutionWhat is the product obtained when sodium benzoate is subjected to decarboxylation ?
View Solution