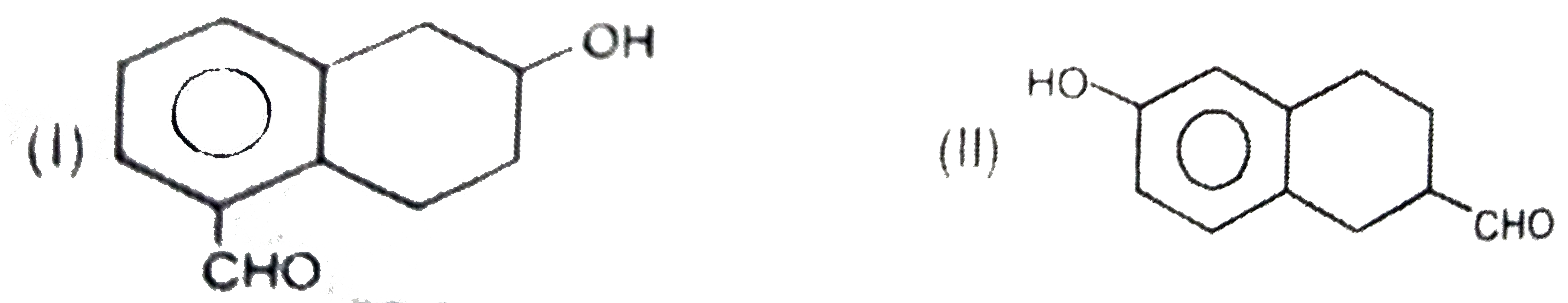
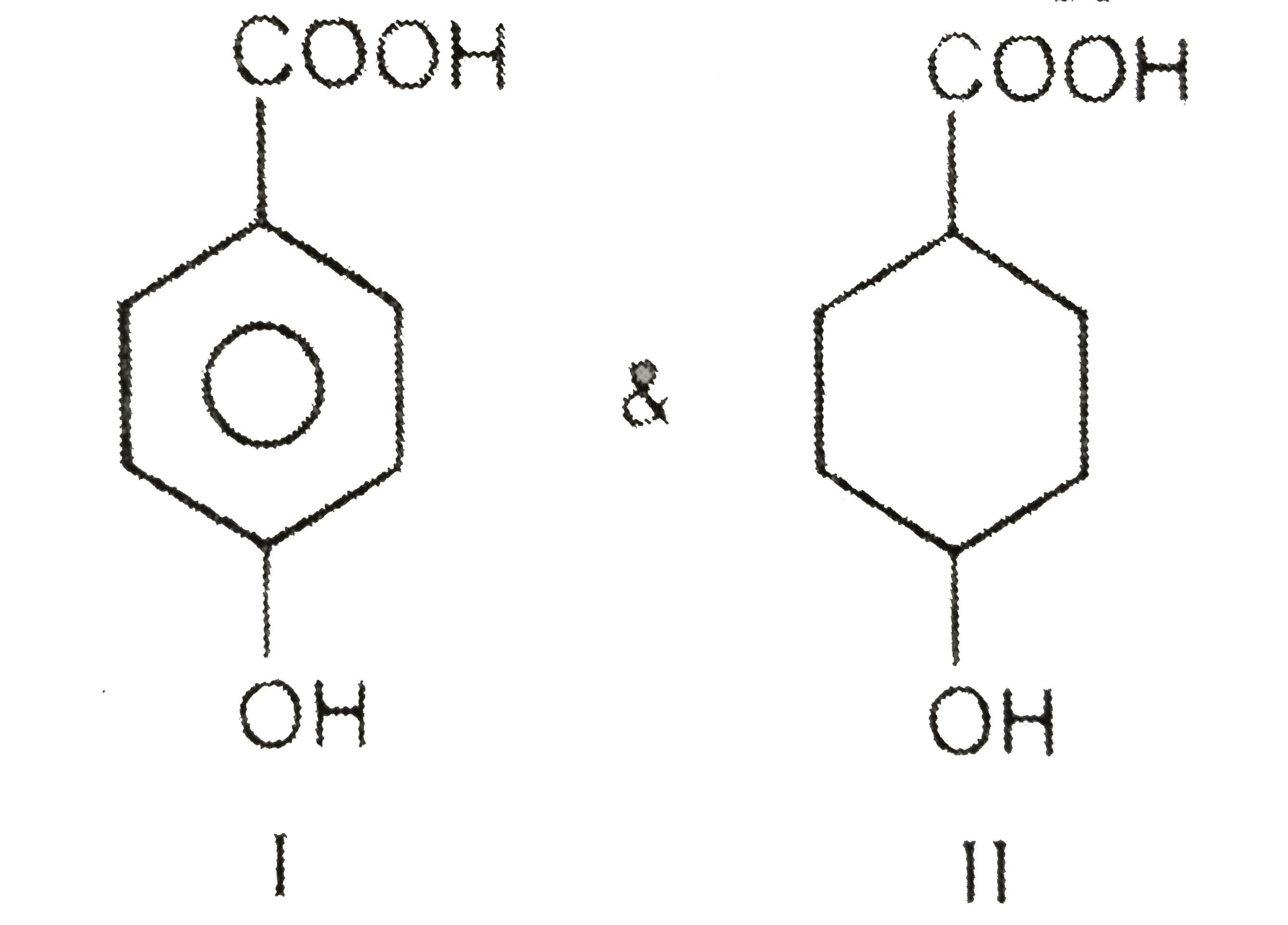
The following two compounds I and II can be distinguished by using reagent
(a) aq. NaHCO3 , Neutral FeCl3
(b) Blue litmus solution , Na metal
(c) HCl/ZnCl2 anhydrous
a or c
b or c
c or d
b or d
The correct Answer is:B
(a) both does not give the test with aq. NaHCO3
(b) It give + ve test with heutral FeCl3 due to presence of phenolic -OH group. But (i) does not .
(c) In (i) aromatic aldehyde does not give
In (I) and (II) acidic hydrogen atom is present so both give +ve test with Na metal.
Topper's Solved these Questions
- STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYEXERCISE - Additional theory
4 Videos
- STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYEXERCISE - Additional Exercise
15 Videos
- STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYEXERCISE - Jee main
6 Videos
- SOLID STATEBOOK - RESONANCE ENGLISHCHAPTER - SOLID STATEEXERCISE - Part- IV
20 Videos
- THERMODYNAMIC & THERMOCHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - THERMODYNAMIC & THERMOCHEMISTRYEXERCISE - INORGANIC CHEMISTRY(P-Block Elements)
25 Videos
Similar Questions
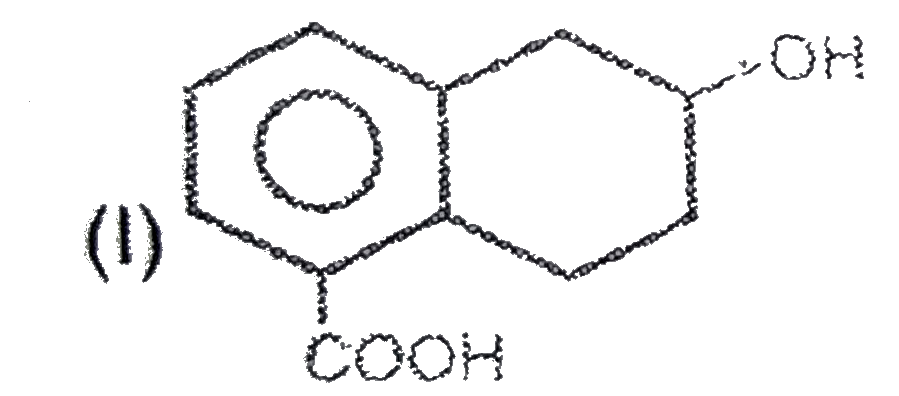
The following two compounds I and II can be distinguished by using reagent
 " "
" " 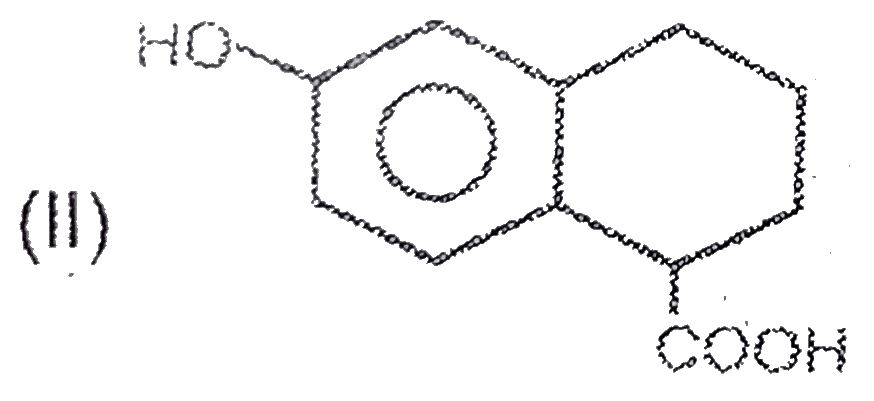
(a) aq. NaHCO3 " " (b) Neutral FeCl3
(c ) Blue litmus solution " " Na metal " " (e) HCl/ZnCl2 anhydrous
View SolutionThe following two compounds I and II can be distinguished by using reagent

(1)Aq.NaHCO3 , (2)Neutral FeCl3(aq.)(FeCl3+NH4OH+H2O)
(3)Blue litmus solution , (4)Na metal
(5)HCl+ZnCl2(anhydrous)
View Solution
Knowledge Check
Which of the following compounds decolourise Br2−H2O and also give positive test with neutral FeCl3 :
A B
B C
C D
D
Which of the following reagent can distinguish the given compound I and II ?
View SolutionAcetaldehyde and Propyne can be distinguish by :
(i) Tollen's reagent " " (ii) l2/NaOH " " (iii) Lucas reagent " " (iv) neutral FeCl3
View SolutionCompounds I and II can be distinguished by using reagent.
I II4-Amino-2methylbut-3-en-2ol4-Amino-2,2-dimethylbut-3-yn-1-ol
View SolutionCompounds I and II can be distinguished by using reagent.
(I)(II)4-Hydroxy-4-methypent-2-enoic acid5-Hydroxypent-2-ynoic acid
View SolutionWhich of the following compounds after mixing can produce blue colouration?
(I) K4[Fe(CN)6]andFeCl3 solution
(II) NH4OHandCuSO4 solution
(III) Adding anhydrous CuSO4 to water
(IV) NH4OH+NiSO4 solution
Choose the correct code:
View SolutionFive isomeric p-subsituted aromatic compounds (A) to (E ) with molecular formula C8H8O2 are given for identifyication. Based on the following observation, give the structures of the compounds.
i. Both (A) and (B) form a silver mirror with Tollens reagent, (B) also gives a positive test with neutral FeCl3 solution.
ii. (C ) gives positive iodoform test.
iii. (D) is readily extracted in aqueous NaHCO3 solution.
iv. (E ) on acid hydrolysis gives 1,4-dihydroxy benzene.
Compound (A) is:
View SolutionThe pair of compounds which cannot exist together in aqueous solution is:
(I)NaH2PO4 and NaHCO3 (II)Na2CO3 and NaHCO3
(III)NaOH and NaH2PO2 (IV)NaHCO3 and NaOH
View Solution

