.72Re162→.z1XA1+ alpha particle
.74W188→.Z2YA2+Beta particle
value of A2−A1Z2−Z1 is :
The correct Answer is:15
To solve the problem, we need to analyze the two nuclear reactions given and extract the values of A1, A2, Z1, and Z2.
Step 1: Analyze the first reaction
The first reaction is:
16272Re→A1Z1X+alpha particle
An alpha particle has a mass number of 4 and an atomic number of 2. Therefore, we can write:
16272Re→A1Z1X+42He
Step 2: Calculate Z1 and A1
Using the conservation of atomic number and mass number:
1. For mass number (A):
162=A1+4⟹A1=162−4=158
2. For atomic number (Z):
72=Z1+2⟹Z1=72−2=70
So, we have:
- A1=158
- Z1=70
Step 3: Analyze the second reaction
The second reaction is:
18874W→A2Z2Y+beta particle
A beta particle (electron) has a mass number of 0 and an atomic number of -1. Therefore, we can write:
18874W→A2Z2Y+0−1e
Step 4: Calculate Z2 and A2
Using the conservation of atomic number and mass number:
1. For mass number (A):
188=A2+0⟹A2=188
2. For atomic number (Z):
74=Z2−1⟹Z2=74+1=75
So, we have:
- A2=188
- Z2=75
Step 5: Calculate the ratio (A2−A1)/(Z2−Z1)
Now we can substitute the values into the ratio:
A2−A1Z2−Z1=188−15875−70
Calculating the numerator and denominator:
- Numerator: 188−158=30
- Denominator: 75−70=5
Thus, the ratio is:
305=6
Final Answer
The value of A2−A1Z2−Z1 is 6.
---
The first reaction is:
16272Re→A1Z1X+alpha particle
16272Re→A1Z1X+42He
Using the conservation of atomic number and mass number:
1. For mass number (A):
162=A1+4⟹A1=162−4=158
72=Z1+2⟹Z1=72−2=70
- A1=158
- Z1=70
The second reaction is:
18874W→A2Z2Y+beta particle
18874W→A2Z2Y+0−1e
Using the conservation of atomic number and mass number:
1. For mass number (A):
188=A2+0⟹A2=188
74=Z2−1⟹Z2=74+1=75
- A2=188
- Z2=75
Now we can substitute the values into the ratio:
A2−A1Z2−Z1=188−15875−70
- Numerator: 188−158=30
- Denominator: 75−70=5
305=6
The value of A2−A1Z2−Z1 is 6.
Topper's Solved these Questions
Similar Questions
If ai>0 for i=1,2,…., n and a1a2 … an=1 , then minimum value of (1+a1)(1+a2) ….. (1+an) is :
View SolutionThe length of projection of the segment join (x1,y1,z1)and(x2,y2,z20 on te line x−αl=y−βm=z−γn is (A) ∣l(x2−x1)+m(y2−y1)+n(z2−z1) (B) |α(x2−x1)+β(y2−y1)+γ(z2−z1)|(C)|(x_2-x_1)/l+(y_2-y_1)/m+(z_2-z_1)/n| (D) none of these
View Solution
Knowledge Check
IfA1andA2 are two AMs between a and b then (2A1−A2)(2A2−A1)=
AabBbaCbaDa2b
Let a1,a2,a3,,a11 be real numbers satisfying a1=15,27−2a2>0andak=2ak−1−ak−2 for k=3,4,,11. If a12+a22+...+a11211=90, then the value of a1+a2++a1111 is equals to _______.
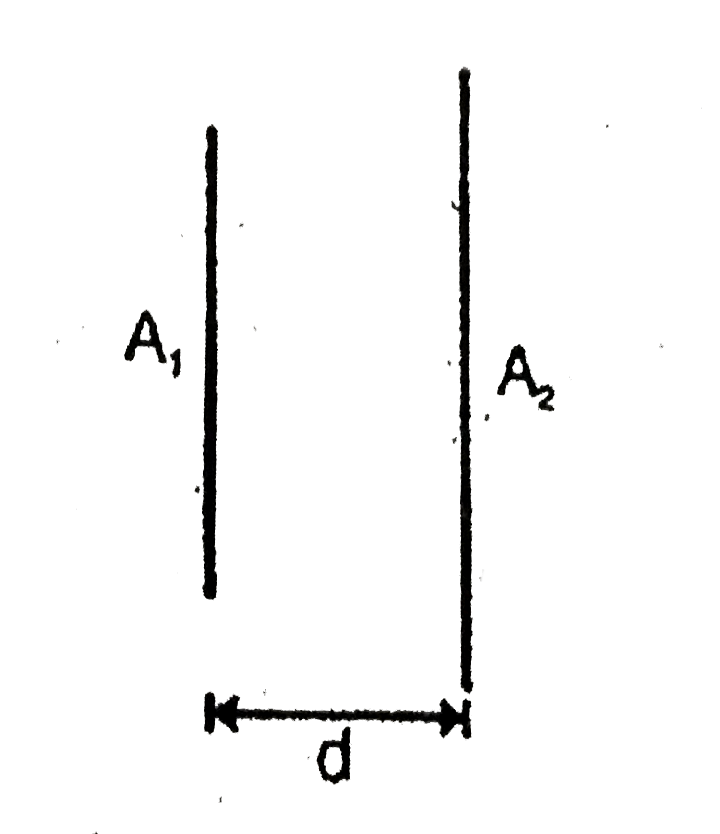
View SolutionThe capacitance of the capacitors of plate areas A1 and A2(A1<A2) at a distance d is
View SolutionIf an>1 for all n∈N then loga2a1+loga3a2+.....loga1an has the minimum value of
View SolutionIf A1 and A2 are two A.M.s between a and b and G1 and G2 are two G.M.s between the same numbers then what is the value of A1+A2G1G2
View SolutionIf the equation of the locus of a point equidistant from the points (a1,b1) and (a2,b2) is (a1−a2)x+(b1−b2)y+c=0 , then the value of c is aa2−a22+b12−b22 √a12+b12−a22−b22 12(a12+a22+b12+b22) 12(a22+b22−a12−b12)
View SolutioncIf a1,a2,a3,..,an∈R then (x−a1)2+(x−a2)2+....+(x−an)2 assumes its least value at x=
View SolutionIf a1,a2,a3,,a2n+1 are in A.P., then a2n+1−a1a2n+1+a1+a2n−a2a2n+a2++an+2−anan+2+an is equal to a. n(n+1)2×a2−a1an+1 b. n(n+1)2 c. (n+1)(a2−a1) d. none of these
View Solution

