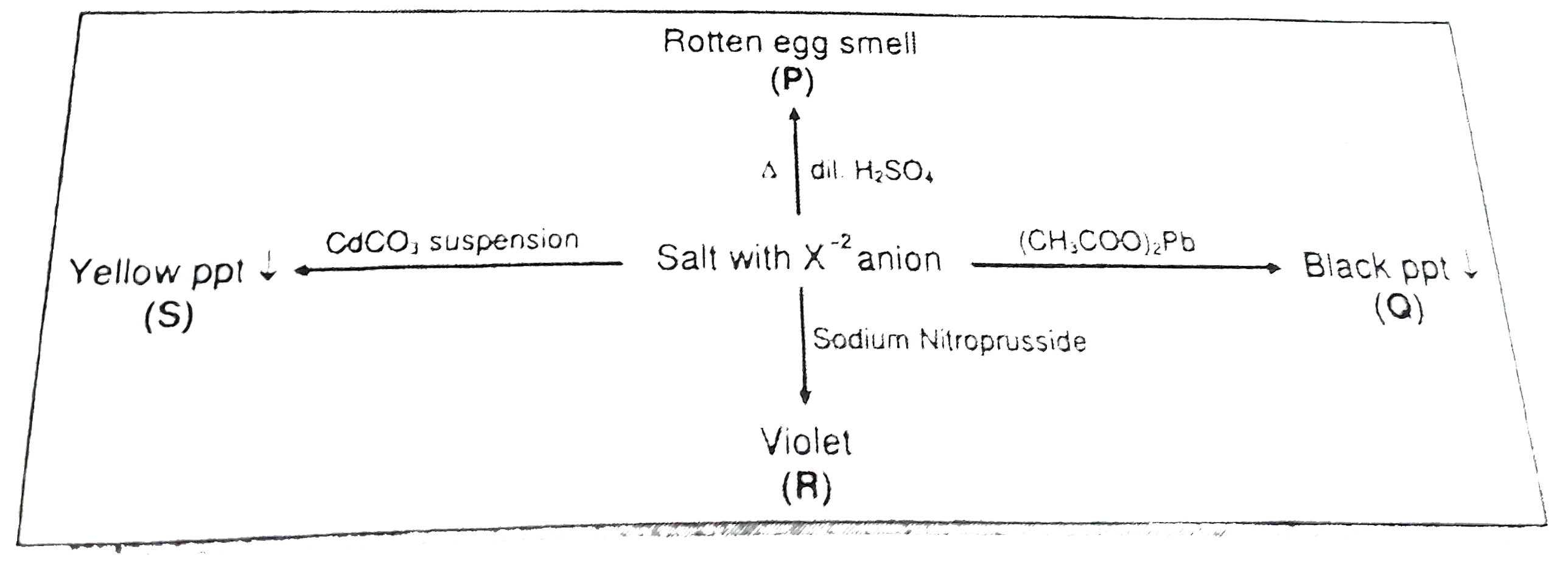
Anion (X2−) is :
CO2−3
SO2−3
S2−
S2O2−3
The correct Answer is:C
Step by step video solution for Anion (X^(2-)) is : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Exercise-2
Explore 44 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Exercise-3
Explore 10 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Matching List Type
Explore 1 Video
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
- QUALITATIVE ANALYSIS PART 1Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS PART 1Exercise:A.L.P
Explore 37 Videos
Similar Questions
Knowledge Check
In the dichromate anion (Cr2O2−7)
Aall Cr - O bonds are equivalentB6 Cr - O bonds are equivalentC3 Cr - O bonds are equivalentDno bonds in Cr2O2−7 are equivalent.
Statement-1 : Electrovalency of oxygen is two (O2−)
Statement-2 : Dinegative anion of oxygen (O2−) is quite common but dinegative anion of sulphur (S2−) is less common.
View SolutionThe radius of a divaent cation A2+ is 94pm and of divalent anion B2− is 146pm. The compound AB has:
View SolutionA salt of NaXMgCl2−−−→ white ppt. on boiling. Thus, anion X is :
View SolutionSalt of Li⊕ with larger anions CO2−3,NOΘ3 are relatively less stable than its salts with small anions. Comment.
View SolutionConsider an ionic solid MX with NaCl structure. Construct a new structure (Z) whose unit cell is constructed from the unit cell of MX following the sequential instructions given below. Neglect the charge balance. 1.Remove all the anions (X) except the central one 2.Replace all the face centered cations (M) by anions (X) 3.Remove all the corner cations (M) 4.Replace the central anion (X) with cation (M)
The value of (number of anion s number of cation s) in z is ________.
View SolutionThe magnetic moment of [NiX4]2− ion is found to be zero. Then the ion is : (X=monodentate anionic ligand)
View SolutionA cation is in the centre touches three anions. Assume that the anions also touch each other. The limiting radius ratio, r+/r− is
View Solution
