The electronic spectrum of [Ti(H2O)6]3+ shows a single broad peak with a maximum at 20,300 cm−1.
The crystal field stabillization energy (CFSE) of the complex ion, in kJ mol−1, is :
(1 kJ mol−1= 83.7 cm−1)
3778
8500
7250
850
The correct Answer is:A
Step by step video solution for The electronic spectrum of [Ti(H_(2)O)_(6)]^(3+) shows a single broad peak with a maximum at "20,300 cm"^(-1). The crystal field stabillization energy (CFSE) of the complex ion, in "kJ mol"^(-1), is : ("1 kJ mol"^(-1) =" 83.7 cm"^(-1)) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Additional Problem for Self Practice (APSP) Part-III Subjected Question
7 Videos
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Additional Problem for Self Practice (APSP) Part-III Only one option correct type
20 Videos
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Additional Problem for Self Practice (APSP) Part-I
29 Videos
- CHEMISTRY IN EVERYDAY LIFEBOOK - RESONANCE ENGLISHCHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - ORGANIC CHEMISTRY(Chemistry in every day life)
31 Videos
- D & F BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - D & F BLOCK ELEMENTSEXERCISE - ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
15 Videos
Similar Questions
What is the coordination number and the oxidation state of the metal in each of the following complexes ?
(a)[ZrF8]4− ,(b)K3[Cr(C2O4)2Cl2]
View SolutionThe correct IUPAC name of the complex is :
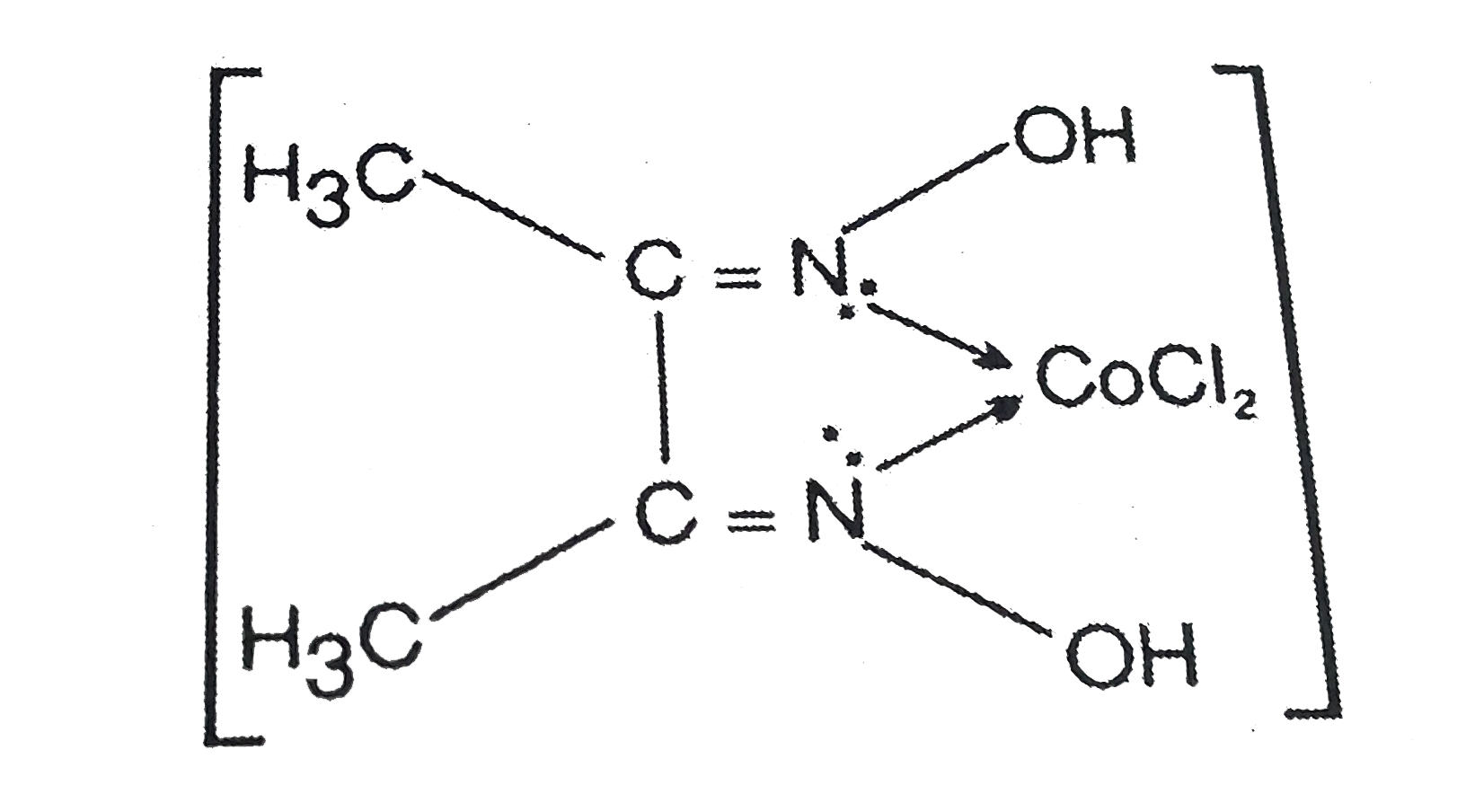
View SolutionIn which of the following complex the nickel metal is in highest oxidation state?
View SolutionWhich is the correct statement about birth control pills?
View SolutionThe number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionCalculate the enthalpy of formation of water, given that the bond energies of H-H, O=O and O-H bond are 433 kJ mol^(-1), 492 kJ mol^(-1) , and 464 kJ mol^(-1) , respectively.
View SolutionCrystal field splitting energy (CFSE) for the complex [Cr(H2O)6]2+ is when P=20925cm−1 and Δ0=10462.5cm−1(1kJmo1−1=83.7cm−1) .
View SolutionThe heat of atomisation of PH_(3)(g) and P_(2)H_(4)(g) are 954 kJ mol^(-1) and 1485 kJ mol^(-1) respectively. The P-P bond energy in kJ mol^(-1) is
View SolutionCalculate resonance energy of C6H6(g).
Given :Δf[C6H6(g)]=−360kJmol−1ΔHSub[C(graphite)]=716kJmol−1B.E.H−H=437kJmol−1B.E.C=C=620kJmol−1B.E.C−C=340kJmol−1B.E.C−H=490kJmol−1
View SolutionThe electronic spectrum of [Ti(H2O)6]3+ shows a single broad peak with a maximum at 20,300 cm−1.
The crystal field stabillization energy (CFSE) of the complex ion, in kJ mol−1, is :
(1 kJ mol−1= 83.7 cm−1)
View SolutionThe electron gain enthalpy ΔegH is -349 kJ mol−1 . If the ground state energy of Cl(g) is x kJ mol−1 . The ground state energy (in kJ mol−1 ) of Cl− (g) is
View SolutionCalculate Delta_(r)H^(@) for the reaction, H_(2)(g) + 1/2O_(2)(g) to H_(2)O(g) Given that bond enthalpies of H-H bond , O = O bond and O - H bond are 433 kJ mol^(-1) , 492 kJ "mol"^(-1) and 464 kJ "mol"^(-1)
View SolutionThe heat of atomisation of PH_(3)(g) and P_(2)H_(4)(g) are 954 kJ "mol"^(-1) and 1485 kJ "mol"^(-1) respectively. The P - P bond energy in kJ "mol"^(-1) is
View SolutionThe standard enthalpies of combustion of C6H6(l),C(graphite)andH2(g) are respectively −3270kJmol−1,−394kJmol−1 and −286kJmol−1. What is the standard enthalpy of formation of C6H6(l) in kJ mol−1?
View SolutionThe heats of atomisation of PH3(g)andP2H4(g) are 954kJmol−1and1485kJmol−1 respectively. The P-P bond energy in kJmol−1 is
View SolutionBelow are some values of enthalpy: O=-141 kJ mol^(-1), F = -328 kJ mol^(-1) S=-200 kJ mol^(-1), Cl=-349 kJ mol^(-1) What values do they represent?
View Solution

