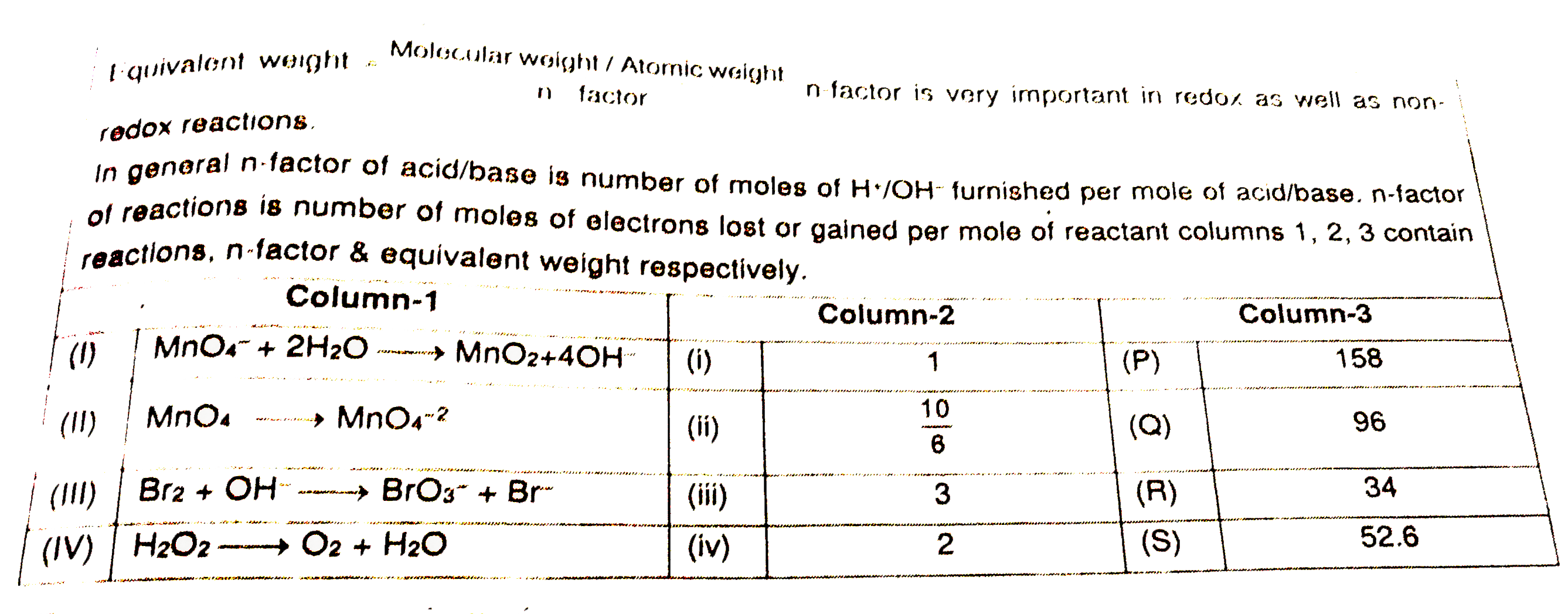
For KMnO4 in neutral medium correct combination is -
(I) (ii) (R)
(II) (ii) (P)
(II) (iii) (S)
(I) (iv) (Q)
The correct Answer is:B
Step by step video solution for For KMnO_(4) in neutral medium correct combination is - by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- EQUIVALENT CONCEPT & TITRATIONSBook:RESONANCE ENGLISHChapter:EQUIVALENT CONCEPT & TITRATIONSExercise:Exercise -3 (Part-I)
Explore 5 Videos
- EQUIVALENT CONCEPT & TITRATIONSBook:RESONANCE ENGLISHChapter:EQUIVALENT CONCEPT & TITRATIONSExercise:Exercise -3 (Part-II)
Explore 7 Videos
- EQUIVALENT CONCEPT & TITRATIONSBook:RESONANCE ENGLISHChapter:EQUIVALENT CONCEPT & TITRATIONSExercise:Exercise -2 (Part-III)
Explore 5 Videos
- ELECTROCHEMISRYBook:RESONANCE ENGLISHChapter:ELECTROCHEMISRYExercise:Advanced Level Problems
Explore 88 Videos
- FUNDAMENTAL CONCEPTBook:RESONANCE ENGLISHChapter:FUNDAMENTAL CONCEPTExercise:ORGANIC CHEMISTRY(Fundamental Concept )
Explore 40 Videos
Similar Questions
In basic medium, I− is oxidised by MnO−4 . In this process, I− changes to :
View SolutionWhen KMnO4 acts as an oxidising agnet and ultimetely from MnO2−4,MnO2,Mn2O3, and Mn2+, then the number of electrons transferred in each case, respectively, are
View SolutionIn the titration of K2Cr2O7 and ferrous sulphate, following data is obtained:
V1 mL of K2Cr2O7 solution of molarity M1 requires V2mL of FeSO4 solution of molarity M2.
Which of the following relations is /are true for the above titration?
View SolutionHow much will the reduction potential of a hydrogen electrode change when its solution initially at pH = 0 is neutralised to pH = 7
View SolutionAmong the following the dissociation constant is highest for
View SolutionThe equivalent weight of KMnO_(4) in (a) neutral medium, (b) acidic medium and (c ) alkaline medium is M//.. ( where M is mol.wt. of KMnO_(4) )
View SolutionDetermine the equivalent weight of the following oxidising and reducing agents : (a) KMnO_(4) (reacting in acidic medium MnO_(4)^(-)rarrMn^(2+) ) (b) KMnO_(4) (reacting in neutral medium MnO_(4)^(-)rarr MnO_(2) )
View Solution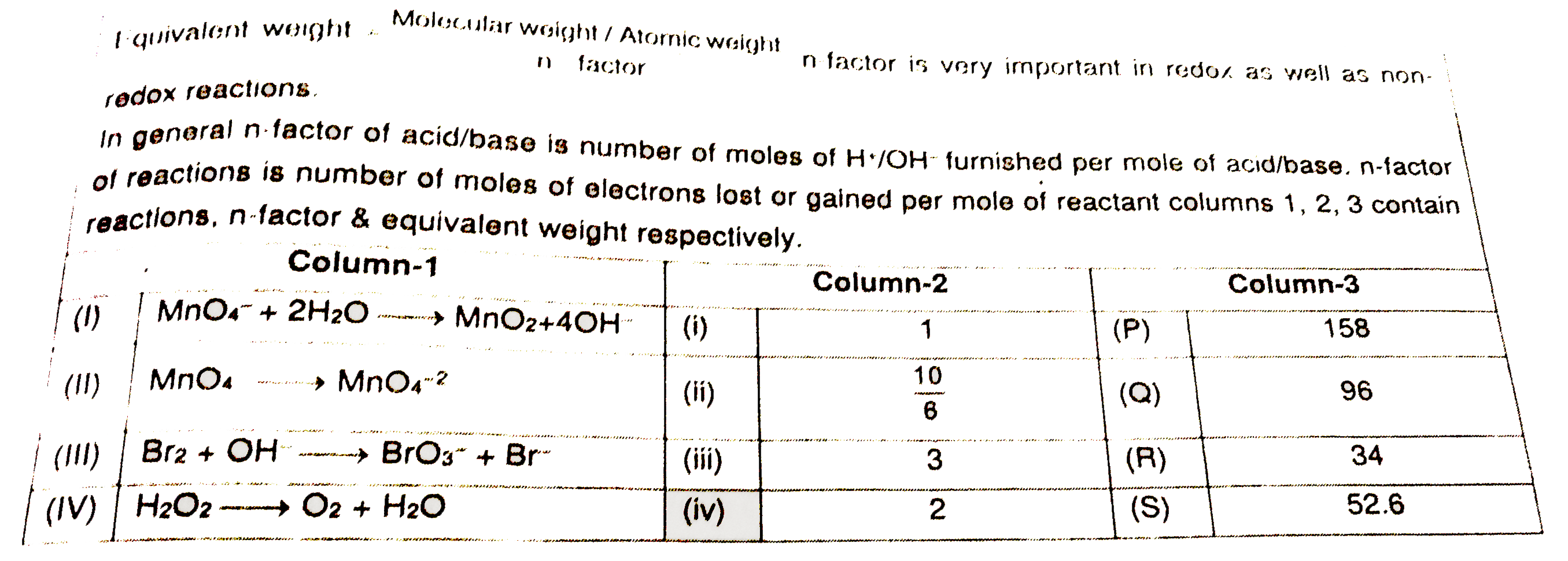
For KMnO4 in strong basic medium correct combination is -
View SolutionFor KMnO_(4) in neutral medium correct combination is -
View SolutionMolecular weight of KMnO_(4) in acidic medium and neutral medium will be respecitvely -
View SolutionWhat is the equivalent weight of KMNO_(4) in: Neutral medium
View SolutionIn neutral or faintly alkaline medium, thiosulphate is quantitatively oxidized by KMnO_(4) is
View Solutionपायरोलुसाइट से KMnO4बनाने की विधि लिखिए तथा KMnO4की अम्लीय,क्षारीय तथा उदासीन माध्यम में ऑक्सीकारक गुणों को एक-एक उदाहरण देकर समझाइए |
View SolutionExplain the oxidising property of KMnO4 in neutral medium .Give the equations.
View Solutionn-Factor of KMnO_(4) in neutral medium is
View SolutionCalculate the equivalent weight of KMnO_(4) in (i) Acidic medium (in) Basic medium (iii) Neutral medium
View Solution
