What does D/L & dl represent.
The correct Answer is:D/L represent nomenclature (relative configuration) while d/l represents direction of optical rotation.
Step-by-Step Solution
1. Understanding D/L and d/l Notation:
- The capital letters D and L are used in the nomenclature of stereoisomers, specifically to denote the configuration of certain molecules, particularly sugars and amino acids.
- The small letters d and l refer to the optical activity of these isomers, indicating the direction in which they rotate plane-polarized light.
2. Capital D and L:
- The designation of D or L is based on the position of the hydroxyl (-OH) group in relation to the chiral carbon furthest from the carbonyl group (C=O) in sugars.
- If the hydroxyl group is on the right side of the chiral carbon, the compound is designated as D (from the Latin "dexter," meaning right).
- If the hydroxyl group is on the left side, it is designated as L (from the Latin "laevus," meaning left).
3. Small d and l:
- The small letters d and l refer to the optical rotation of the isomers.
- A compound that rotates plane-polarized light to the right (clockwise) is designated as d (positive rotation).
- A compound that rotates plane-polarized light to the left (counterclockwise) is designated as l (negative rotation).
4. Relative Nature of D/L and d/l:
- It is important to note that D and L are relative terms. You cannot refer to a single isomer as D or L without a counterpart.
- Similarly, d and l also require a comparison with another isomer to establish their optical activity.
5. Example:
- For instance, in a sugar molecule, if we have two isomers, one with the hydroxyl group on the right (D) and one with it on the left (L), we can then refer to their optical activities as d (if it rotates light to the right) and l (if it rotates light to the left).
- The capital letters D and L are used in the nomenclature of stereoisomers, specifically to denote the configuration of certain molecules, particularly sugars and amino acids.
- The small letters d and l refer to the optical activity of these isomers, indicating the direction in which they rotate plane-polarized light.
- The designation of D or L is based on the position of the hydroxyl (-OH) group in relation to the chiral carbon furthest from the carbonyl group (C=O) in sugars.
- If the hydroxyl group is on the right side of the chiral carbon, the compound is designated as D (from the Latin "dexter," meaning right).
- If the hydroxyl group is on the left side, it is designated as L (from the Latin "laevus," meaning left).
- The small letters d and l refer to the optical rotation of the isomers.
- A compound that rotates plane-polarized light to the right (clockwise) is designated as d (positive rotation).
- A compound that rotates plane-polarized light to the left (counterclockwise) is designated as l (negative rotation).
- It is important to note that D and L are relative terms. You cannot refer to a single isomer as D or L without a counterpart.
- Similarly, d and l also require a comparison with another isomer to establish their optical activity.
- For instance, in a sugar molecule, if we have two isomers, one with the hydroxyl group on the right (D) and one with it on the left (L), we can then refer to their optical activities as d (if it rotates light to the right) and l (if it rotates light to the left).
टॉपर्स ने हल किए ये सवाल
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART 1 SECTION (H))
2 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART 1 SECTION (I))
6 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART 1 SECTION (F))
4 Videos
- SOLUTIONSBOOK - RESONANCE ENGLISHCHAPTER - SOLUTIONSEXERCISE - Advabced Level Problems (PART-2)
35 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Advanced level Problems (Part-III)
12 Videos
समान प्रश्न
Which of the following are chiral compound.
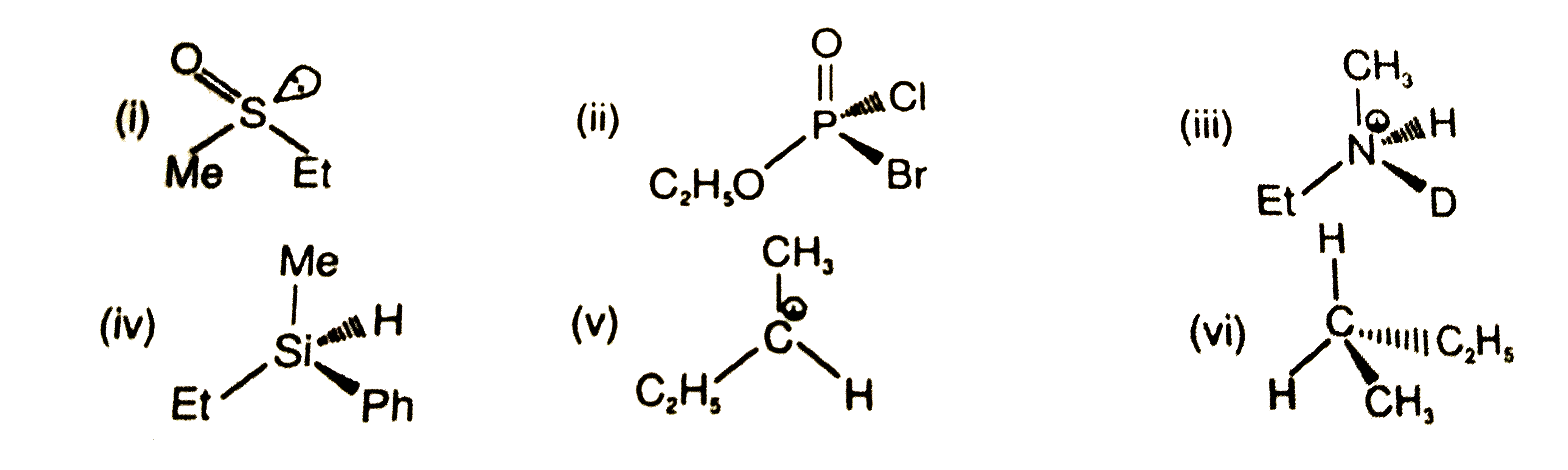
View SolutionFind the number of geometrical isomers possible of the following compounds.

View SolutionIdentify the pairs of enantiomers and diastereomers from the following:
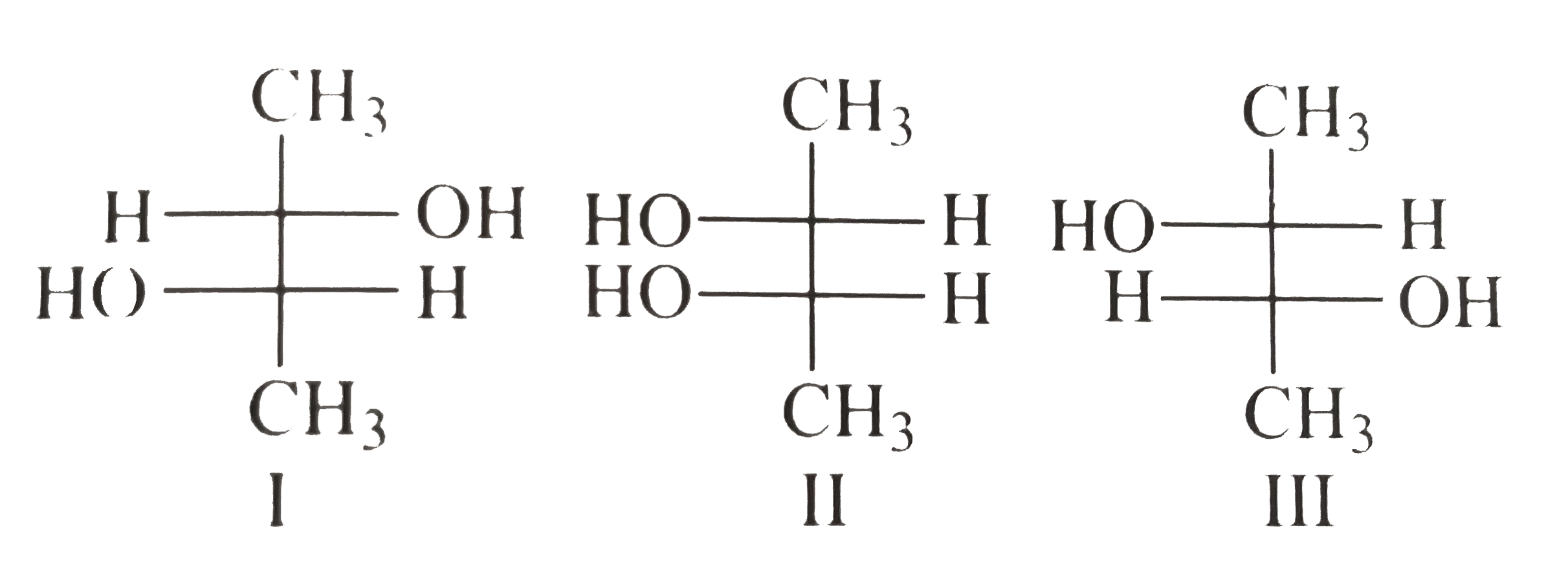
View SolutionThe osmotic pressure of 6% solution of urea at 300 K is
View SolutionWhat simple laboratory test could be performed to distinguish between 1-pentyne and 2-pentyne?
View SolutionWhat does D/L & dl represent.
View Solutionसमीकरण τ=dLdt में, यदि τ बल आघूर्ण है तो L का मात्रक क्या hoga ?
View SolutionWhat does a wave represent ?
View SolutionThe letter D and L in carbohydrates represent
View Solutionm = D/t किसको दर्शाता है?
View Solutionఈ గుర్తు దేనిని సూచిస్తుంది

View SolutionΔT क्या प्रदर्शित करता है -
View SolutionΔ P क्या प्रदर्शित करता है-
View SolutionWhat does a modem represent?
View Solutionthe value of ∮¯¯¯B.¯¯¯dl for the loop L is
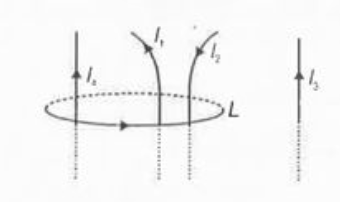
View SolutionConsider the following two equations
A)L=Iω (B)dLdt=τ
View Solution

