Total number of optically stereoisomers of CH3−C∣CIH−C∣CIH−C∣CIH−CH3
2
4
6
8
The correct Answer is:A
To determine the total number of optically active stereoisomers for the compound CH₃−CH(CI)−CH(CI)−CH₃, we will follow these steps:
Step 1: Identify the Structure
The given compound can be represented as:
CH₃|CH−CI|CH−CI∣CH₃
This structure indicates that there are multiple carbon atoms, some of which are bonded to chlorine (Cl) atoms.
Step 2: Identify Chiral Centers
Chiral centers (or stereogenic centers) are carbon atoms that are bonded to four different groups. In this compound, we need to analyze each carbon atom:
1. The two terminal carbons (attached to CH₃ groups) are not chiral because they are bonded to three hydrogen atoms.
2. The two central carbons (each bonded to a Cl, a hydrogen, and a CH group) are chiral centers because they are bonded to four different groups:
- For the first chiral center: Cl, H, and the two CH groups.
- For the second chiral center: Cl, H, and the two CH groups.
Step 3: Count the Chiral Centers
From the analysis, we find that there are 2 chiral centers in the compound.
Step 4: Use the Formula for Optical Stereoisomers
The number of optically active stereoisomers can be calculated using the formula:
\( \text{Number of stereoisomers} = 2^n \)
where \( n \) is the number of chiral centers.
Substituting the value of \( n \):
\( \text{Number of stereoisomers} = 2^2 = 4 \)
Conclusion
Thus, the total number of optically active stereoisomers for the compound is 4.
---
The given compound can be represented as:
CH₃|CH−CI|CH−CI∣CH₃
This structure indicates that there are multiple carbon atoms, some of which are bonded to chlorine (Cl) atoms.
Chiral centers (or stereogenic centers) are carbon atoms that are bonded to four different groups. In this compound, we need to analyze each carbon atom:
2. The two central carbons (each bonded to a Cl, a hydrogen, and a CH group) are chiral centers because they are bonded to four different groups:
- For the first chiral center: Cl, H, and the two CH groups.
- For the second chiral center: Cl, H, and the two CH groups.
From the analysis, we find that there are 2 chiral centers in the compound.
The number of optically active stereoisomers can be calculated using the formula:
\( \text{Number of stereoisomers} = 2^n \)
where \( n \) is the number of chiral centers.
\( \text{Number of stereoisomers} = 2^2 = 4 \)
Thus, the total number of optically active stereoisomers for the compound is 4.
টপার এই প্রশ্নগুলো সমাধান করেছে
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART II SECTION (J))
11 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART II SECTION (K))
6 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART II SECTION (H))
4 Videos
- SOLUTIONSBOOK - RESONANCE ENGLISHCHAPTER - SOLUTIONSEXERCISE - Advabced Level Problems (PART-2)
35 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Advanced level Problems (Part-III)
12 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
The IUPAC name ofCH3−OH∣C∣H−CH2−O∣∣C−CH3 is
A2-Hydroxypentan-4-oneB4-Hydroxypentan-2-oneC4-oxopentan-2-olD2-oxopentan-4-olThe IUPAC name of CH3−O∣∣C−C∣C2H5H−C∣CH3H−CH3 is
A3-(Methylethyl) pentan-2-oneB3-(Methylethyl)pentan-4-oneC3-Ethyl-4-methylpentan-2-oneD3-Ethyl-2-methylpentan-4-one
Number of chiral carbon persent in the following compound:
CH3−C∣OHH−CH2−C∣BrH−C∣C2H5H−CH3
View SolutionTotal number of stereoisomers of compound CH3−CH=CH−C∣CIH−CH=CH−CH3 are :
View SolutionWrite the product formed in the following reaction :
Ph−CH3∣C∣OH−C∣OHH−C∣OHH−CH3∣CH−CH2OHHIO4(excess)−−−−−−−−→
View SolutionDesignate the species (A) and (B) as electrophile ( E) or nucleophile (Nu) in the following reactions :
(a) BEt3+Me2..O:→Me2⊕O..−ΘBEt3
(b) CH2=O+:ΘSO3H→Bisulphite ionCH2∣OΘ−SO3H
( c) C_2H_5C1 + :CH:^(Ө) rarrC_2H_5 -CN + C1^(Ө)
(d)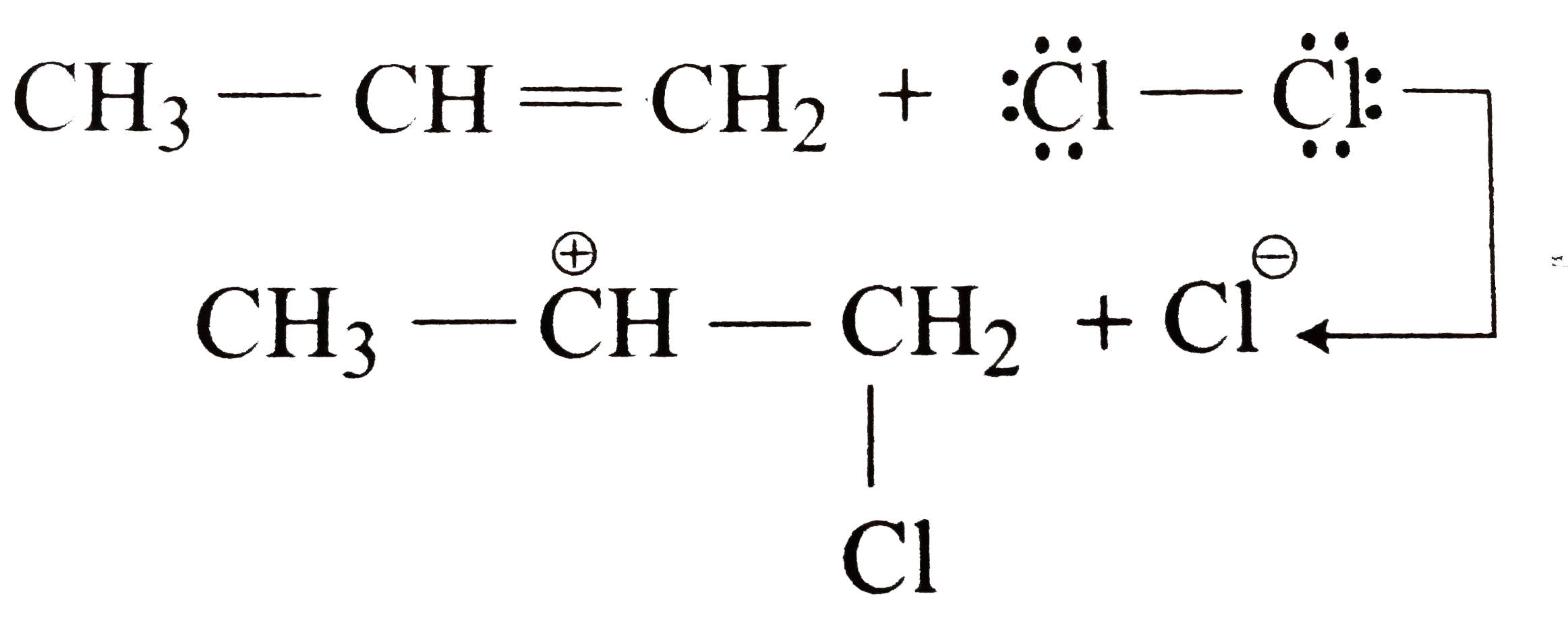
( e) CH3−⊕CH−CH2∣CI+CIΘ→CH3−CH∣CI−CH2∣CI
(f) :⊕N..H2+CH3−C≡CH→CH3−C≡C..Θ+NH3
View SolutionThe number of tertiary carbon atoms in the following^ structure is CH3−CH3∣C∣CH3−CH2−CH3∣CH−CH3
View SolutionCH3−CH3∣C∣CH3−CH=CH−CH3H−Cl−−−→ ?
View Solution

