Which pair is identical ?

The correct Answer is:A
To determine which pair of compounds is identical, we will analyze each pair systematically based on their structural configurations and stereochemistry.
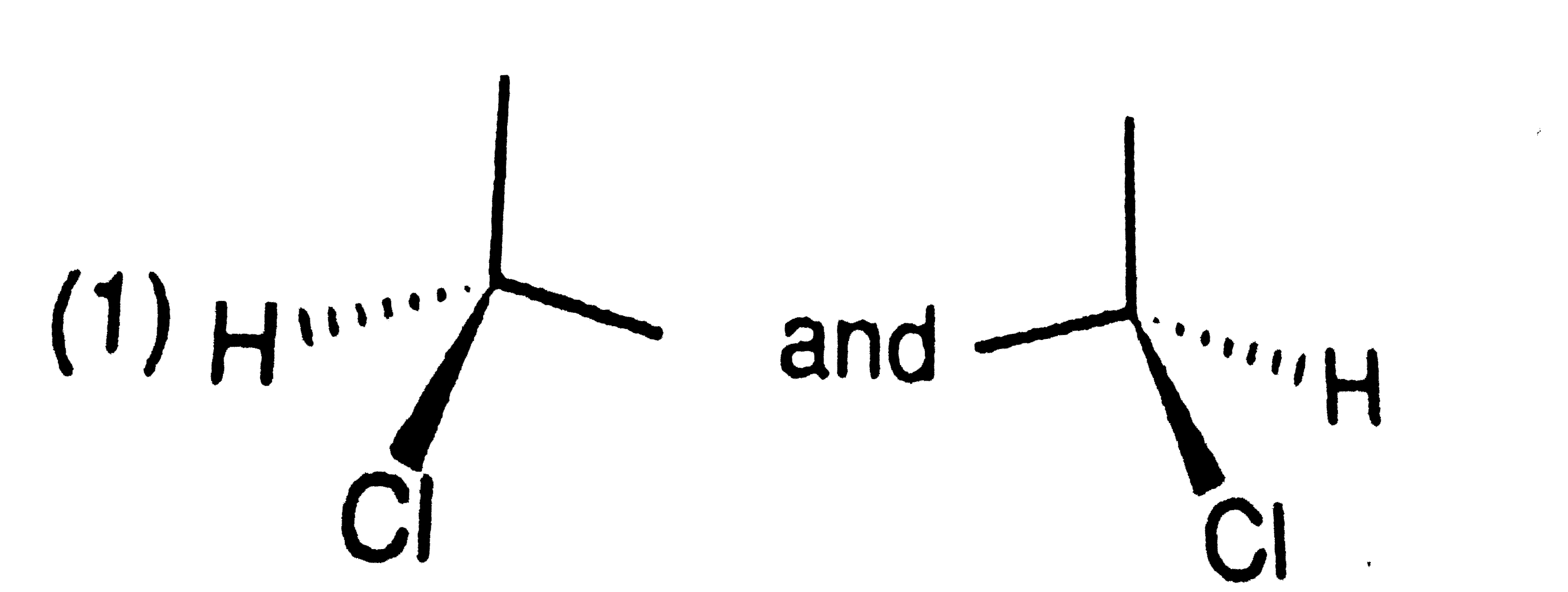
1. Identify the First Pair:
- The first compound has two methyl groups attached to the same carbon.
- Since there are no chiral centers in this molecule, it is symmetrical.
- Thus, both structures are identical.
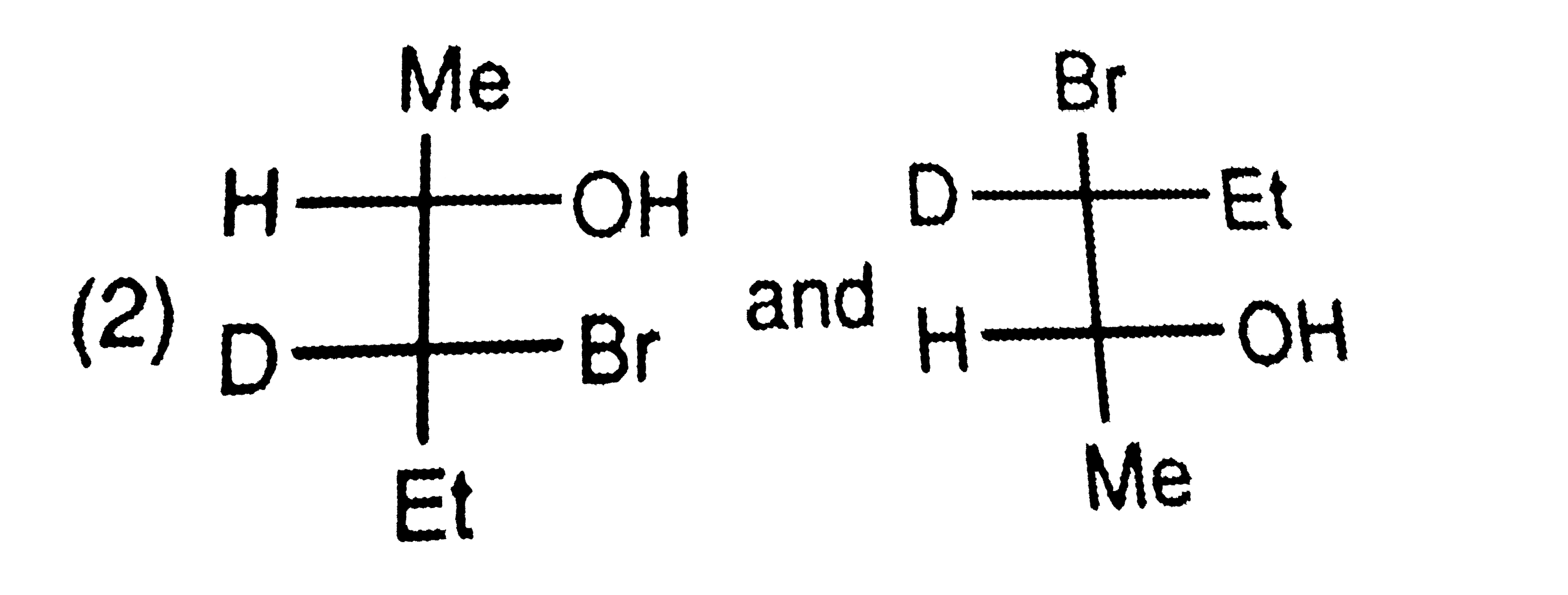
2. Analyze the Second Pair:
- The second compound has a hydroxyl group (OH), a bromine (Br), and an ethyl group (Et) attached to two different carbon centers.
- We need to determine the R/S configuration for both chiral centers.
- For the first carbon:
- Assign priorities: OH (1), Br (2), CH3 (3), H (4).
- The configuration is clockwise (R) when H is in the back, but since H is in front, we flip it to S.
- For the second carbon:
- Assign priorities: Br (1), OH (2), Et (3), H (4).
- The configuration is counterclockwise (S) when H is in the back, but since H is in front, we flip it to R.
- Since the configurations are different (R and S), these compounds are not identical.
3. Examine the Third Pair:
- The third compound has two hydroxyl groups and we need to analyze their configurations.
- If we rotate one of the molecules by 180 degrees, we find that the positions of the OH groups swap.
- This results in an enantiomer relationship, indicating that they are not identical.
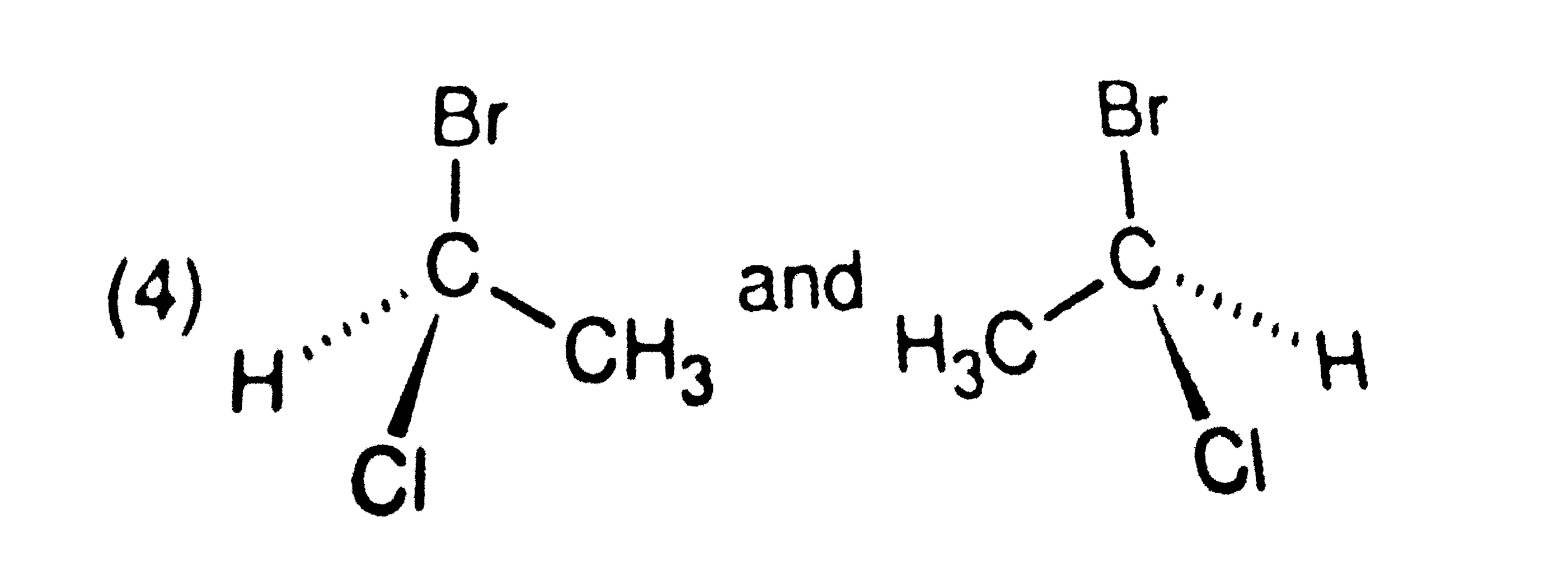
4. Evaluate the Fourth Pair:
- The fourth compound also has two chiral centers.
- For the first carbon:
- Assign priorities: Br (1), Cl (2), CH3 (3), H (4).
- The configuration is counterclockwise (S).
- For the second carbon:
- Assign priorities: Br (1), Cl (2), CH3 (3), H (4).
- The configuration is clockwise (R).
- Since one is S and the other is R, these compounds are also not identical.
Conclusion:
The only pair that is identical is the first pair of compounds. The other pairs are either enantiomers or different molecules.
---
- The first compound has two methyl groups attached to the same carbon.
- Since there are no chiral centers in this molecule, it is symmetrical.
- Thus, both structures are identical.
- The second compound has a hydroxyl group (OH), a bromine (Br), and an ethyl group (Et) attached to two different carbon centers.
- We need to determine the R/S configuration for both chiral centers.
- For the first carbon:
- Assign priorities: OH (1), Br (2), CH3 (3), H (4).
- The configuration is clockwise (R) when H is in the back, but since H is in front, we flip it to S.
- For the second carbon:
- Assign priorities: Br (1), OH (2), Et (3), H (4).
- The configuration is counterclockwise (S) when H is in the back, but since H is in front, we flip it to R.
- Since the configurations are different (R and S), these compounds are not identical.
- The third compound has two hydroxyl groups and we need to analyze their configurations.
- If we rotate one of the molecules by 180 degrees, we find that the positions of the OH groups swap.
- This results in an enantiomer relationship, indicating that they are not identical.
- The fourth compound also has two chiral centers.
- For the first carbon:
- Assign priorities: Br (1), Cl (2), CH3 (3), H (4).
- The configuration is counterclockwise (S).
- For the second carbon:
- Assign priorities: Br (1), Cl (2), CH3 (3), H (4).
- The configuration is clockwise (R).
- Since one is S and the other is R, these compounds are also not identical.
The only pair that is identical is the first pair of compounds. The other pairs are either enantiomers or different molecules.
Topper's Solved these Questions
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART II : NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-1)
55 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
23 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART -II ONLINE JEE MAIN)
3 Videos
- SOLUTIONSBOOK - RESONANCE ENGLISHCHAPTER - SOLUTIONSEXERCISE - Advabced Level Problems (PART-2)
35 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Advanced level Problems (Part-III)
12 Videos
Similar Questions
Knowledge Check
Which of the following pairs have identical bond order?
AF2andO2−2BN2andCO2CO2andO−2DN2andN+2
Which pair is correct?
View SolutionChoose the pairs having identical value of van't Hoff factor
View SolutionWhich pair is of insectivorous plants
View SolutionWhich pair is correctly matched ?
View SolutionWhich pair is an example of anomers?
View SolutionWhich of the following pairs have identical value of bond order?
View SolutionWhich of the following pairs have identical value of bond order?
View Solution

