Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
In the form of elemental nitrogen it exists as a diatomic molecule (N2). This is due to the fact that nitrogen can form pπ−pπ multiple bond (N≡N) because of small of nitrogen atom. Heavier elements of this group do not able to form pπ−pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Further P−P single bond is stronger than N−N single bond. Hence phosphorus as tendency to under go catenation
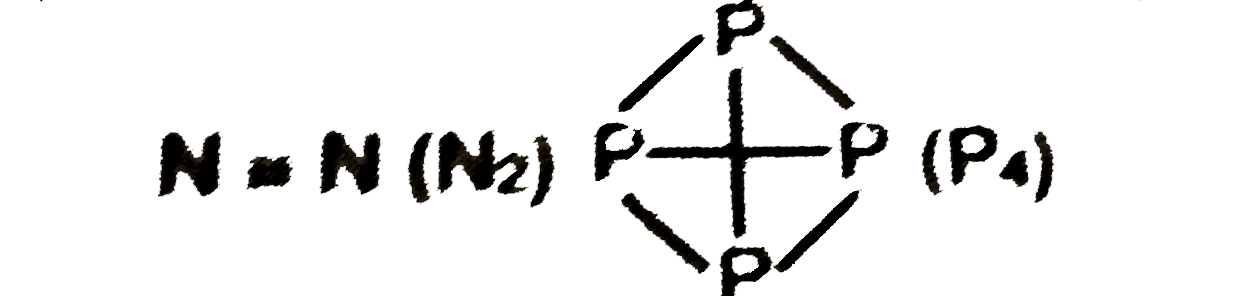
Topper's Solved these Questions
- NITROGEN & OXYGEN FAMILYBOOK - RESONANCE ENGLISHCHAPTER - NITROGEN & OXYGEN FAMILYEXERCISE - Exercise 3 (Part-II: JEE MAIN)
13 Videos
- NITROGEN & OXYGEN FAMILYBOOK - RESONANCE ENGLISHCHAPTER - NITROGEN & OXYGEN FAMILYEXERCISE - Exercise 3 (JEE (MAIN) Online problems)
11 Videos
- NITROGEN & OXYGEN FAMILYBOOK - RESONANCE ENGLISHCHAPTER - NITROGEN & OXYGEN FAMILYEXERCISE - Exercise 2 (Part : IV Comprehension)
13 Videos
- METALLURGYBOOK - RESONANCE ENGLISHCHAPTER - METALLURGYEXERCISE - ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)
17 Videos
- NITROGEN CONTAINING COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - NITROGEN CONTAINING COMPOUNDSEXERCISE - ORGANIC CHEMISTRY(Nitrogen containing Compounds)
30 Videos
Similar Questions
The number of hydrgen atom(s) attached to phosphours atom in hypophosphorus acid is :
View SolutionWhich of the following is not formed when H2S reacts acidic K2Cr2O7 solution ?
View SolutionNitrogen forms the largest number of oxides as it is capable of forming stable multiple bonds with oxygen.They range from N2O(O.S of nitrogen+1) through NO,N2O3,NO2,N2O4 to N2O5(O.S of nitrogen +5).
Following points are important regarding the study of oxides of nitrogen.
(a)All oxides of nitrogen except N2O6 and endothermic as a large amount of energy is required to dissociate the stable molecule of oxygen and nitrogen.
(b)The small electronegativity difference between oxygen and nitrogen make N-O bond easily breakable to give oxygen and hence oxides of nitrogen are said to be better oxidising agents.
(c )Except N2O5, all are gases at ordinary temperature. N2O3 is stable only at lower temperature (253 K).
(d) Except N2O and NO which are neutral oxides, all are acidic oxides which dissolve in water forming corresponding oxy acids.
(e)They are also good example for illustrating the concept the resonance.
The gas which is acidic in nature is :
View SolutionIn purification of bauxite ore, it is mixed with coke and heated at 1800∘C in presence of nitrogen, this is :
View SolutionThe positive carbylamine test is not given by
View SolutionNitrogen exists as diatomic molecule and phosphorus as P_4 . Why?
View SolutionNitrogen exists as diatomic molecule and phosphorus as P_(4) . Why ?
View SolutionNitrogen exists as diatomic molecule (N_(2)) while phosphorus as tetra-atomic molecule (P_(4)) . Why ?
View SolutionExplain why nitrogen exists as a diatomic molecule and phosphorus at P_(4) molecule.
View Solutionनाइट्रोजन दिपरमाणुक अणु के रूप में पाया जाता हैं। तथा फॉस्फोरस P4 के रूप में । क्यों?
View SolutionNitrogen exists as diatomic molecule and phosphorus as P_(4) . Why ?
View Solutionनाइट्रोजन द्विपरमाणुक अणु के रूप में पाया जाता है तथा फास्फोरस P4 के रूप में । क्यों ?
View SolutionNitrogen exists as diatomic molecule and phosphorus as P_(4) - Why ?
View SolutionNitrogen exists as diatomic molecule and phosphorus as P_(4) . Why ?
View Solutionकारण स्पष्ट कीजिए (संक्षेप में)
(i) नाइट्रोजन द्विपरमांडुक अणु के रूप में पाया जाता है तथा फॉस्फोरस P4 के रूप में क्यों?
View SolutionNitrogen exists as diatomic molecule and phosphorus as P_(4) . Why?
View Solution

