Gases which turns lime water milky are;
The correct Answer is:A, B, C
Step-by-Step Solution:
1. Identify Lime Water: Lime water is a dilute solution of calcium hydroxide, which is represented by the chemical formula Ca(OH)2.
2. Understanding the Reaction with Carbon Dioxide: When carbon dioxide (CO2) is bubbled through lime water, it reacts with calcium hydroxide to form calcium carbonate (CaCO3), which is a white precipitate. The reaction can be represented as:
Ca(OH)2+CO2→CaCO3↓+H2O
The formation of CaCO3 causes the lime water to turn milky.
3. Understanding the Reaction with Sulfur Dioxide: When sulfur dioxide (SO2) is bubbled through lime water, it reacts with calcium hydroxide to form calcium sulfite (CaSO3), which is also a white precipitate. The reaction can be represented as:
Ca(OH)2+SO2→CaSO3↓+H2O
This also results in the lime water turning milky.
4. Conclusion: Therefore, the gases that turn lime water milky are carbon dioxide (CO2) and sulfur dioxide (SO2).
Final Answer: The gases which turn lime water milky are CO2 (carbon dioxide) and SO2 (sulfur dioxide).
---
Ca(OH)2+CO2→CaCO3↓+H2O
The formation of CaCO3 causes the lime water to turn milky.
Ca(OH)2+SO2→CaSO3↓+H2O
This also results in the lime water turning milky.
Topper's Solved these Questions
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID BOOK - RESONANCE ENGLISHCHAPTER - CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID EXERCISE - Exercise-2 Part-I
1 Video
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID BOOK - RESONANCE ENGLISHCHAPTER - CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID EXERCISE - Part-I
98 Videos
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID BOOK - RESONANCE ENGLISHCHAPTER - CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID EXERCISE - Part-II
64 Videos
- BIOMOLECULES & POLYMERBOOK - RESONANCE ENGLISHCHAPTER - BIOMOLECULES & POLYMEREXERCISE - ORGANIC CHEMISTRY(Biomolecules & Polymer)
34 Videos
- CHEMICAL KINETICSBOOK - RESONANCE ENGLISHCHAPTER - CHEMICAL KINETICSEXERCISE - PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)
49 Videos
Similar Questions
In the given reaction
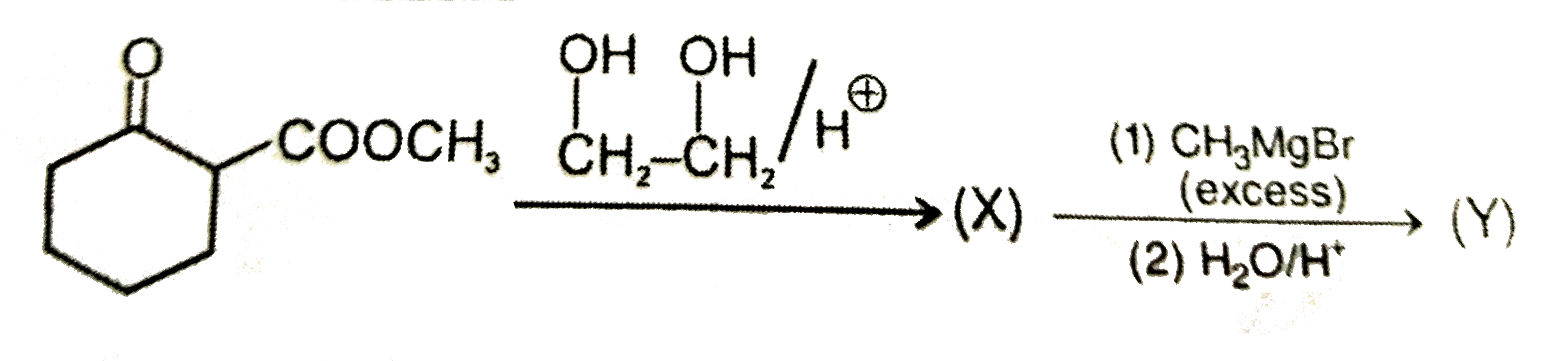 (Y) is
(Y) is
View Solution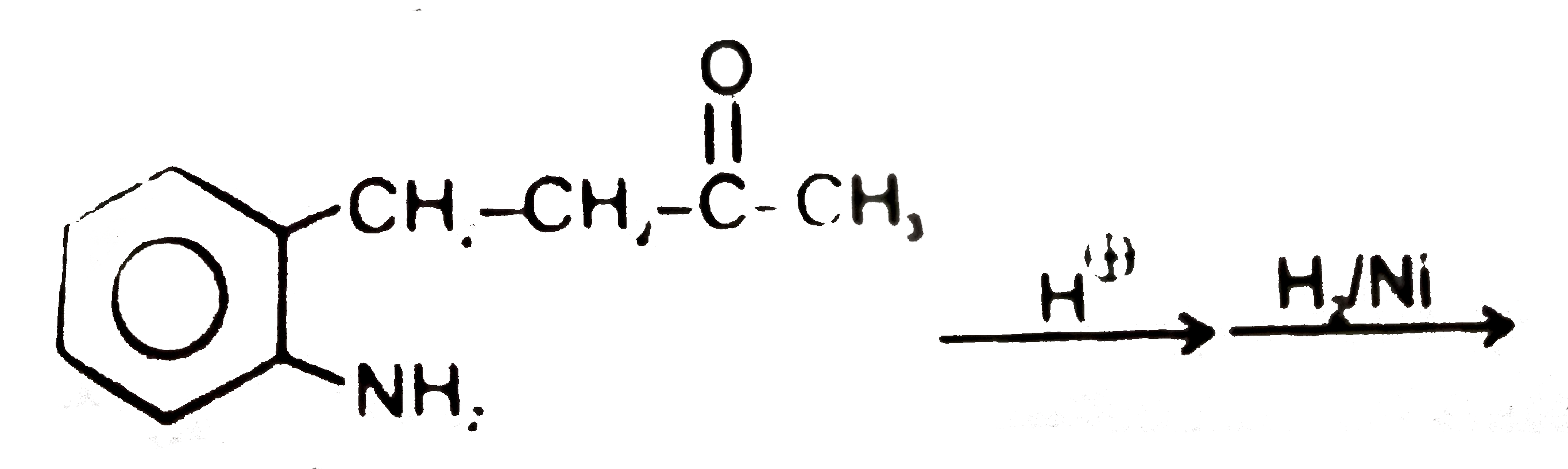
View SolutionA mixed salt of calcium acetate formate on dry distillation gives
View SolutionWhich two of the following aldohexoses give the same osazone derivative ?
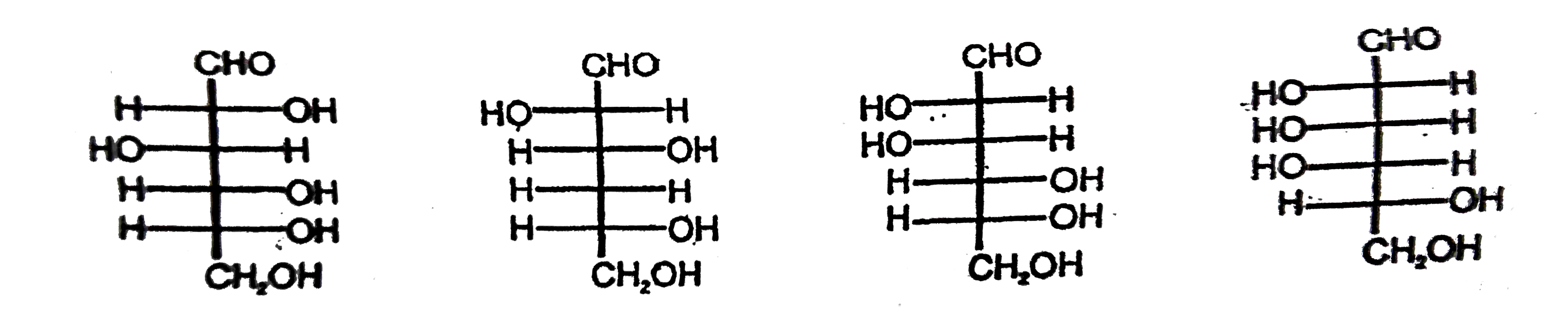
View SolutionThe half-life of decomposition of gaseous CH3CHO at initial pressure of 365mm and 170mm of Hg were 420sec and 880sec respectively. The order of the reaction is:
View SolutionThe characteristics of some gases are given . Explain how they cause pollution.
(a) A colourless , odourless gas which turns lime water milky.
(b) A colourless , pungent gas which turns lime water milky.
View SolutionWhich of the following gases turn lime water milky when passed throught it.
View SolutionMark the gas which turns lime water milky
View SolutionWhich of the following gases turn lime water milky?
View SolutionWhich gas turns lime water milky ?
View SolutionWhich gas turns lime water milky ?
View SolutionWhich gas in this experiment turns the lime water milky ?
View SolutionWhich gas turns lime water milky?
View SolutionWhich gas turns lime water milky?
View SolutionThe gas which turns lime water milky is :
View Solution

