A compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. The number of amino groups present per molecule of the former compound is
2
5
4
6
The correct Answer is:B
Step by step video solution for A compound with molecular mass 180 is acylated with CH_(3)COCl to get a compound with molecular mass 390. The number of amino groups present per molecule of the former compound is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Book:RESONANCE ENGLISHChapter:CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Exercise:Part-III
Explore 8 Videos
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Book:RESONANCE ENGLISHChapter:CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Exercise:Exercise-2 Part-I
Explore 1 Video
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Book:RESONANCE ENGLISHChapter:CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Exercise:Exercise-1 Part-II
Explore 1 Video
- BIOMOLECULES & POLYMERBook:RESONANCE ENGLISHChapter:BIOMOLECULES & POLYMERExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 34 Videos
- CHEMICAL KINETICSBook:RESONANCE ENGLISHChapter:CHEMICAL KINETICSExercise:PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)
Explore 49 Videos
Similar Questions
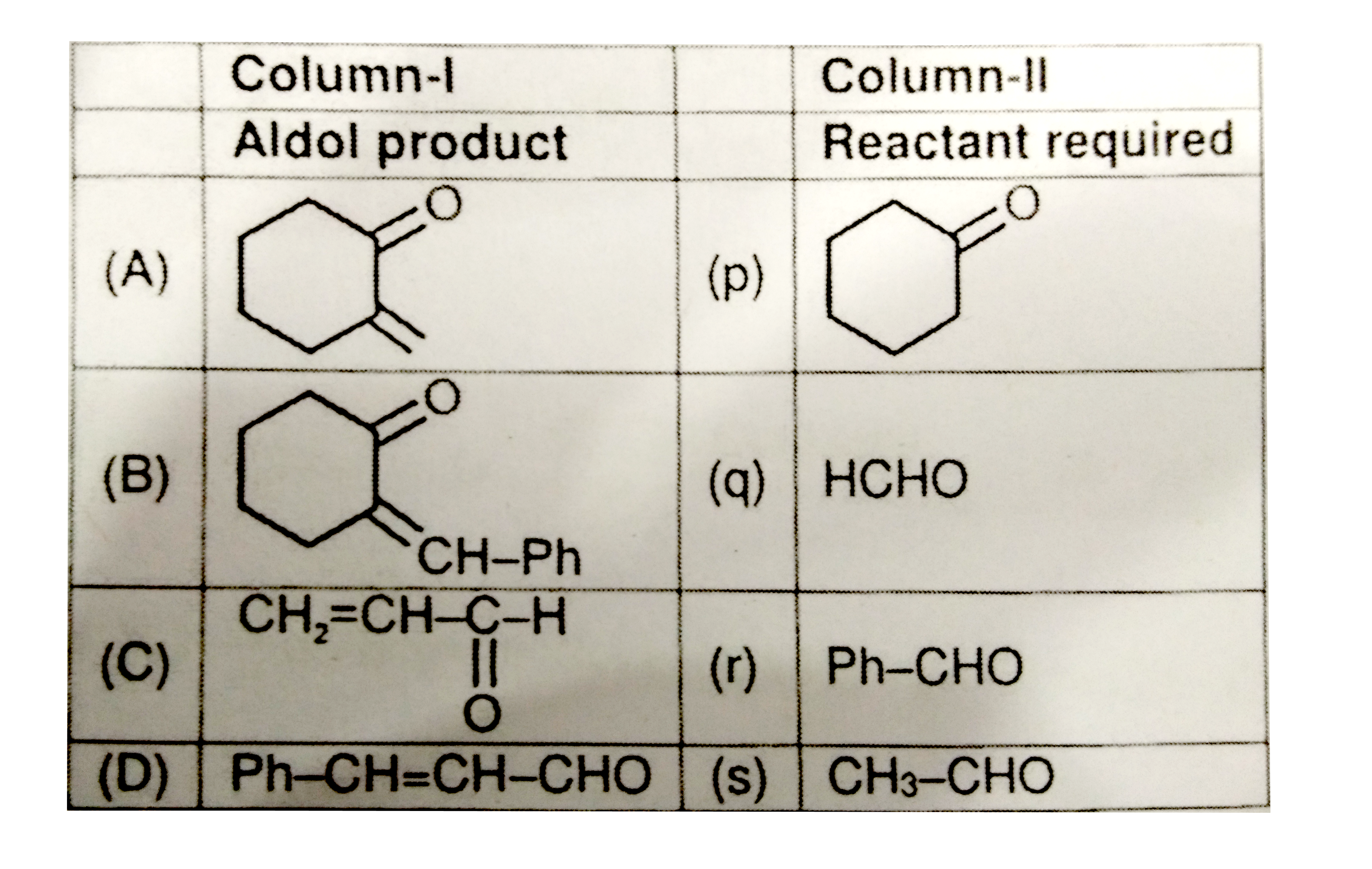
Match the coloumn
View SolutionIn the given reaction
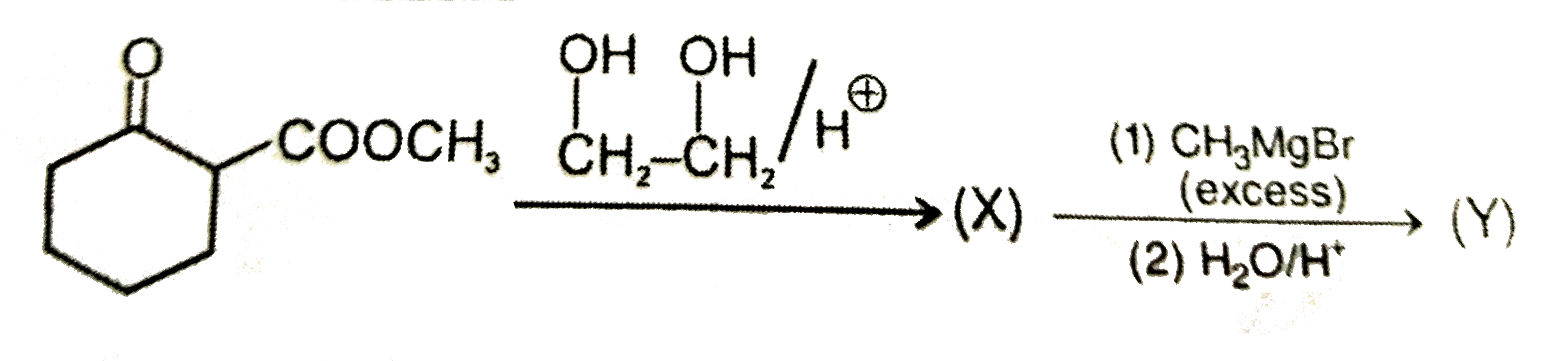 (Y) is
(Y) is
View SolutionOn heating calcium propionate, the product formed is
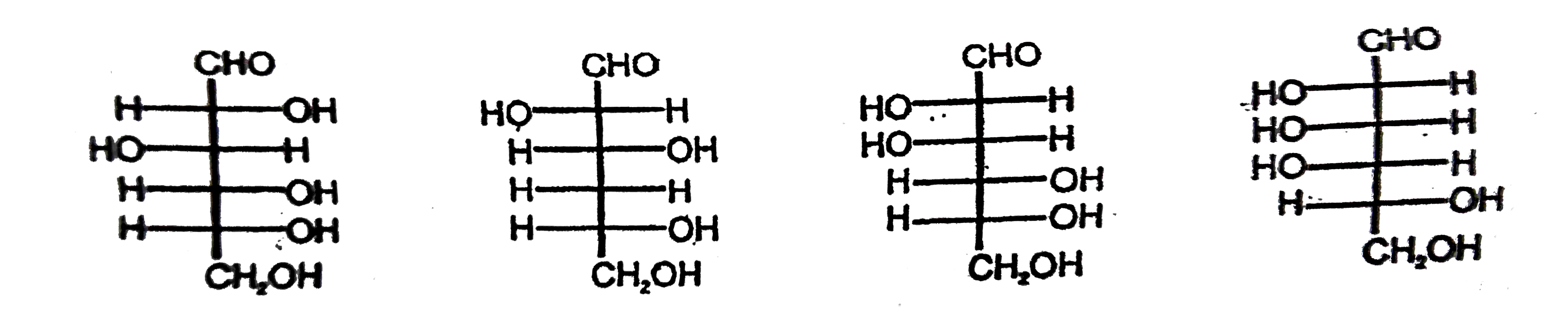
View SolutionWhich two of the following aldohexoses give the same osazone derivative ?
View SolutionThe half-life of decomposition of gaseous CH3CHO at initial pressure of 365mm and 170mm of Hg were 420sec and 880sec respectively. The order of the reaction is:
View SolutionThe compound with mol. Mass 180 is acetylated with CH3COCl to get acetylated compound with mol. Mass 390. The number of amino groups present per molecule of the compound is
View SolutionA compound with mlecular mass 180 is acylated with CH_(3)COCl to get a compound with molecular mass 390. The number of amino group present per molecule of the former compound is
View SolutionCH3COCl is used to detect the number of amino groups in the organic compounds. When a compound of molecular mass 180 is acetylated, gives a compound of molecular mass 390. The number of amino groups in the compound is
View SolutionA compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. the number of amino groups present per molecules of the former compounds is
View Solutionएक यौगिक जिसका अणुभार 180 हो, CH3COCl के साथ ऐसीटिलीकृत करके एक यौगिक बनाया गया जिसका अणुभार 390 है। पहले यौगिक के प्रति अणु में उपस्थित ऐमीनों समूहों की संख्या है:
View SolutionA compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. The number of amino groups present per molecule of the former compound is :
View SolutionA compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. the number of amino groups present per molecule of the former compound is
View SolutionA compound with molecular mass 180 is acylated with CH_(3)COCl to get a compound with molecular mass 390. the number of amio groups present per molecule of the former compound is
View SolutionA compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. the number of amino groups present per molecule of the former compound is-
View SolutionAn organic compound (P) having molecular mass 180 is acylated with CH3COCl to get a new compound with molecular mass 348. The number of amino groups present per molecule of compound (P) is
View SolutionA compound with molecular mass 180 is acylated with CH_(3)COCl to get a compound with molecular mass 390. The number of amino groups present per molecule of the former compound is :
View Solution
