Identify final product In the following reaction CH3−C∣OHH−COOHΔ−−→ product
CH2≡CH−COOH
CH3−C∣∣O−COOH
CH3−CH2−OH
The correct Answer is:A
To identify the final product of the given reaction, we will analyze the reactant and the conditions under which the reaction occurs. The reactant is a compound containing both a carboxylic acid group and a hydroxyl group. Let's break down the steps:
Step 1: Identify the Reactant
The reactant is given as CH₃-CH(OH)-C(=O)OH, which is a carboxylic acid with a hydroxyl group.
Step 2: Understand the Reaction Conditions
The reaction is carried out under heating (Δ), which indicates that the reaction is likely to involve dehydration (loss of water).
Step 3: Dehydration Reaction
When a carboxylic acid reacts with a hydroxyl group under heat, a dehydration reaction occurs. In this case, the hydroxyl group (–OH) from the carboxylic acid and a hydrogen atom (H) from the hydroxyl group will be removed, leading to the formation of water (H₂O).
Step 4: Formation of the Final Product
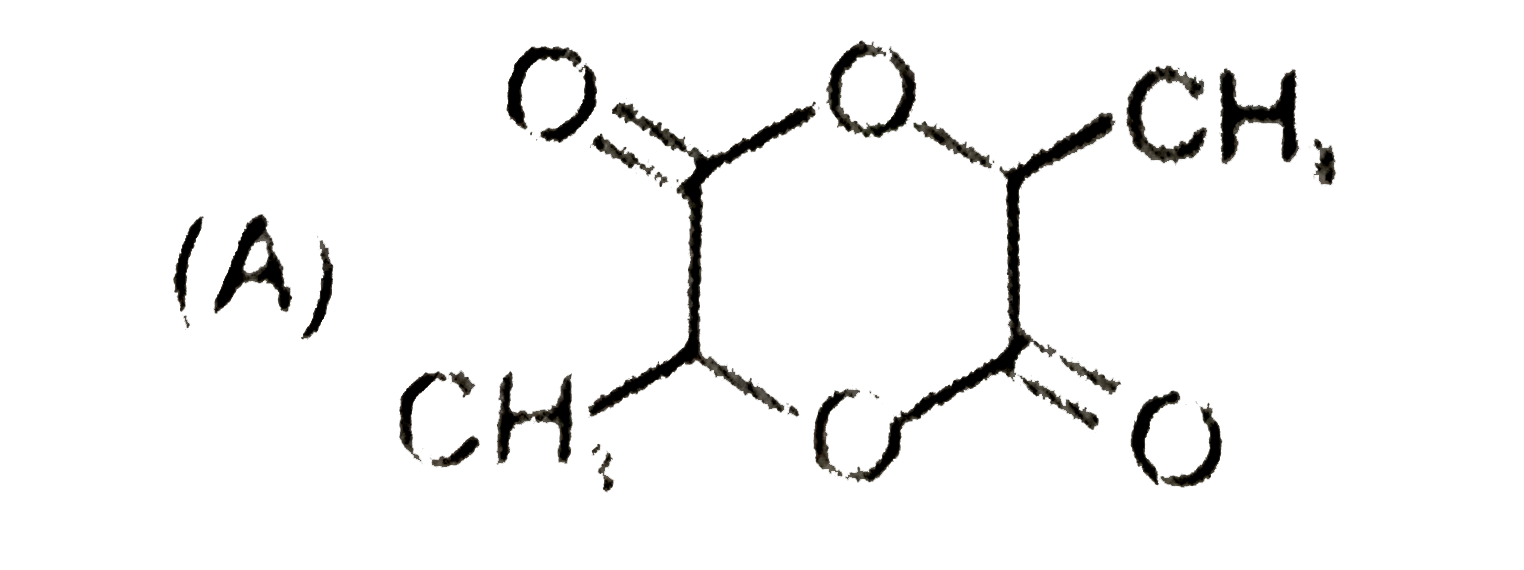
The remaining parts of the molecule will then combine to form a cyclic compound. The dehydration will result in the formation of a cyclic ester (also known as a lactone). The structure will involve a carbonyl group (C=O) and an ether linkage (–O–) in a ring.
Step 5: Drawing the Final Product
The final product can be represented as follows:
- The cyclic structure will have an oxygen atom in the ring and will connect two carbon atoms that were originally part of the carboxylic acid and hydroxyl group.
- The final product will be a cyclic compound with the structure:
OCC/\CC
Conclusion
The final product of the reaction is a cyclic compound (lactone) formed by the dehydration of the original reactant.
Final Product
The final product is a cyclic ester (lactone) formed from the dehydration of the reactant.
---
The reactant is given as CH₃-CH(OH)-C(=O)OH, which is a carboxylic acid with a hydroxyl group.
The reaction is carried out under heating (Δ), which indicates that the reaction is likely to involve dehydration (loss of water).
When a carboxylic acid reacts with a hydroxyl group under heat, a dehydration reaction occurs. In this case, the hydroxyl group (–OH) from the carboxylic acid and a hydrogen atom (H) from the hydroxyl group will be removed, leading to the formation of water (H₂O).
The remaining parts of the molecule will then combine to form a cyclic compound. The dehydration will result in the formation of a cyclic ester (also known as a lactone). The structure will involve a carbonyl group (C=O) and an ether linkage (–O–) in a ring.
The final product can be represented as follows:
- The cyclic structure will have an oxygen atom in the ring and will connect two carbon atoms that were originally part of the carboxylic acid and hydroxyl group.
- The final product will be a cyclic compound with the structure:
The final product of the reaction is a cyclic compound (lactone) formed by the dehydration of the original reactant.
The final product is a cyclic ester (lactone) formed from the dehydration of the reactant.
টপার এই প্রশ্নগুলো সমাধান করেছে
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Book:RESONANCE ENGLISHChapter:CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Exercise:Part -III
Explore 30 Videos
- BIOMOLECULES & POLYMERBook:RESONANCE ENGLISHChapter:BIOMOLECULES & POLYMERExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 34 Videos
- CHEMICAL KINETICSBook:RESONANCE ENGLISHChapter:CHEMICAL KINETICSExercise:PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)
Explore 49 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Identify final product In the following reaction CH3−C∣OHH−COOHΔ−−→ product
A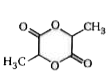 BCH2=CHCO2HCCH3−O∣∣CCO2HDCH3CH2OH
BCH2=CHCO2HCCH3−O∣∣CCO2HDCH3CH2OH
Consider the following reactions
I.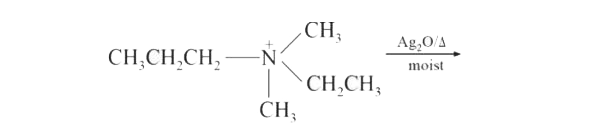
II.(CH3)2C∣OH−CH2−CHOΔ−−→
III. (CH3)2CHCH2−C∣FH−CH3CH3O−Na+−−−−−−−−−−→Δ
IV. (CH3)2CH−C∣BrH−CH3(CH3)3CO−k+−−−−−−−−−−→
Which of these reaction will not produce Hoffmann product?
View SolutionProducts of the following reaction are
CH3−C∣CH3=CH−CH3KMnO4/H−−−−−−−−−→
View SolutionWhat is the product of the following reaction : CH3−C∣CH3=CH2HBr−−−−→Peroxide
View SolutionWhat would be the major products in the following reactions:
CH3−CH3∣C∣CH3−CH2BrC2H5OH−−−−→Δ
View SolutionWhat would be the major products in the following reactions:
CH3−CH3∣C∣CH3−CH2BrC2H5OH−−−−→Δ
View SolutionWhat would be the major products in the following reactions:
CH3−CH3∣C∣CH3−CH2BrC2H5OH−−−−→Δ
View SolutionThe major product of the following reaction is:
CH3−CH3∣C∣H−CHCH3∣BrCH3OH−−−−→
View Solution
