Write the reactions involved in preparation of phenol from benzene sulfonic acid.
To prepare phenol from benzene sulfonic acid, we follow a series of chemical reactions. Here’s a step-by-step breakdown of the process:
Step 1: Formation of Sodium Salt of Benzene Sulfonic Acid
1. Starting Material: Benzene sulfonic acid (C6H5SO3H).
2. Reaction: Treat benzene sulfonic acid with an aqueous solution of sodium hydroxide (NaOH).
C6H5SO3H+NaOH→C6H5SO3Na+H2O
- Product: Sodium salt of benzene sulfonic acid (C6H5SO3Na).
Step 2: Formation of Sodium Phenoxide
3. Reaction: The sodium salt of benzene sulfonic acid is then fused with solid sodium hydroxide (NaOH) at a high temperature (around 350°C).
C6H5SO3Na+NaOHΔ−→C6H5O−Na+Na2SO3+H2O
- Product: Sodium phenoxide (C6H5O^-Na).
Step 3: Acidification to Form Phenol
4. Reaction: The sodium phenoxide is then acidified with dilute sulfuric acid (H2SO4).
C6H5O−Na+H2SO4→C6H5OH+Na2SO4
- Product: Phenol (C6H5OH) and sodium sulfate (Na2SO4) as a by-product.
Summary of Reactions
1. Benzene sulfonic acid + NaOH → Sodium salt of benzene sulfonic acid + H2O
2. Sodium salt of benzene sulfonic acid + NaOH (heat) → Sodium phenoxide + Na2SO3 + H2O
3. Sodium phenoxide + H2SO4 → Phenol + Na2SO4
Final Product
- The final product obtained is phenol (C6H5OH).
---
1. Starting Material: Benzene sulfonic acid (C6H5SO3H).
2. Reaction: Treat benzene sulfonic acid with an aqueous solution of sodium hydroxide (NaOH).
C6H5SO3H+NaOH→C6H5SO3Na+H2O
- Product: Sodium salt of benzene sulfonic acid (C6H5SO3Na).
3. Reaction: The sodium salt of benzene sulfonic acid is then fused with solid sodium hydroxide (NaOH) at a high temperature (around 350°C).
C6H5SO3Na+NaOHΔ−→C6H5O−Na+Na2SO3+H2O
- Product: Sodium phenoxide (C6H5O^-Na).
4. Reaction: The sodium phenoxide is then acidified with dilute sulfuric acid (H2SO4).
C6H5O−Na+H2SO4→C6H5OH+Na2SO4
- Product: Phenol (C6H5OH) and sodium sulfate (Na2SO4) as a by-product.
1. Benzene sulfonic acid + NaOH → Sodium salt of benzene sulfonic acid + H2O
2. Sodium salt of benzene sulfonic acid + NaOH (heat) → Sodium phenoxide + Na2SO3 + H2O
3. Sodium phenoxide + H2SO4 → Phenol + Na2SO4
- The final product obtained is phenol (C6H5OH).
Similar Questions
Knowledge Check
The reaction used for the preparation of phenol is
A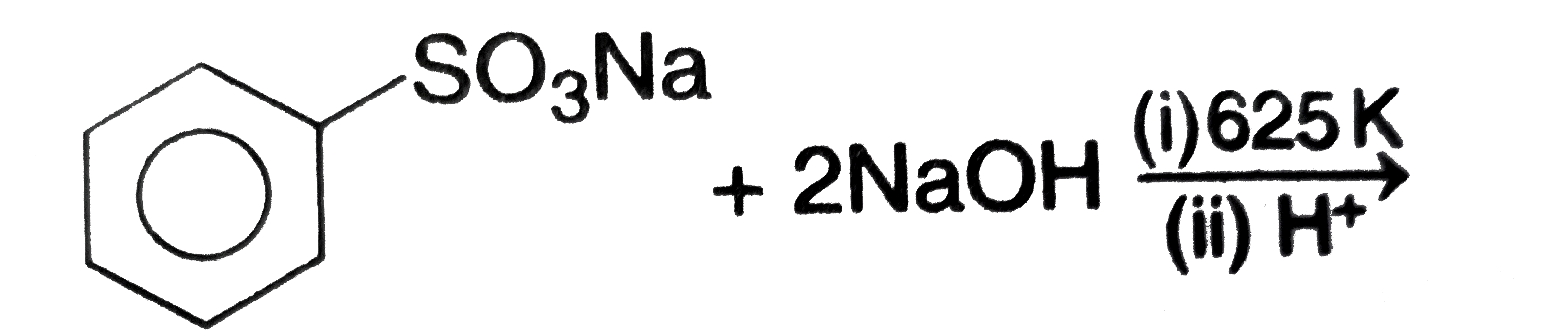 B
B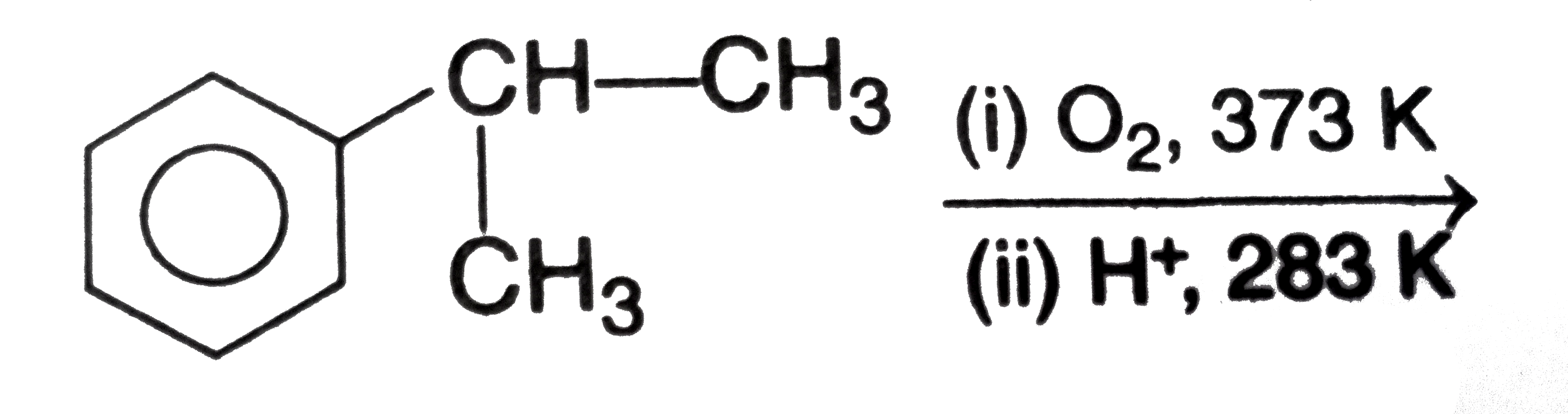 C
C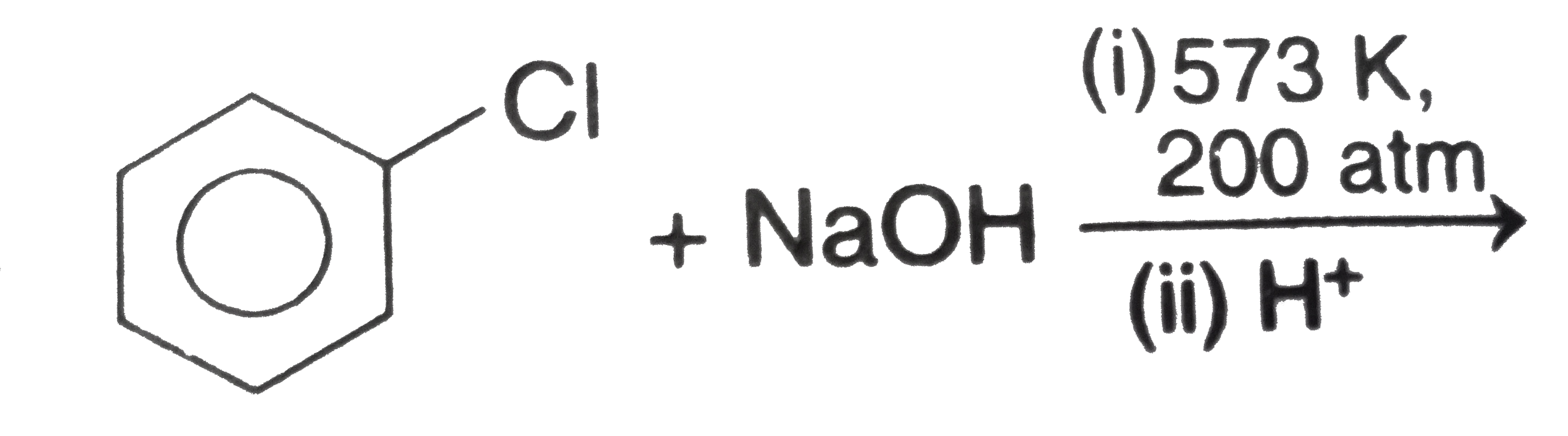 DAll of the above
DAll of the aboveWhich reaction is involved in the preparation of ethene from chloroethane?
Aα eliminationBβeliminationCreductionDnone of the above
Write chemical reaction for the preparation of phenol from chlorobenzene.
View SolutionName the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
View SolutionName the enzymes and write the reactions involved in the preparation of ethanol from sucrose by fermentation.
View SolutionWrite the reactions involved in the preparation of :
(a) Teflon (b) Orlon (c) PVC
View SolutionWrite the reaction involved in large scale preparation of niric acid.
View SolutionWrite the chemical reaction for the preparation of phenol form chlorobenzene.
View Solution
