Correct order of acid strength for
i)acetic acid ii) fluoroacetic acid iii) 4-Nitrobenzoic acid iv) 4-Methyl benzoic acid is……….
igt iigt iii gt iv
i gt iii gt iv gt i,
iii gt iv gt i gt ii
iv gt i gt ii gt iii
To determine the correct order of acid strength among acetic acid, fluoroacetic acid, 4-nitrobenzoic acid, and 4-methylbenzoic acid, we need to analyze the stability of their conjugate bases after the dissociation of the proton (H⁺). The more stable the conjugate base, the stronger the acid.
1. Identify the Compounds and Their Conjugate Bases:
- Acetic Acid (CH₃COOH): Upon losing H⁺, it forms the conjugate base CH₃COO⁻.
- Fluoroacetic Acid (CHF₂COOH): Upon losing H⁺, it forms the conjugate base CHF₂COO⁻.
- 4-Nitrobenzoic Acid (C₆H₄(NO₂)COOH): Upon losing H⁺, it forms the conjugate base C₆H₄(NO₂)COO⁻.
- 4-Methylbenzoic Acid (C₆H₄(CH₃)COOH): Upon losing H⁺, it forms the conjugate base C₆H₄(CH₃)COO⁻.
2. Analyze the Effects of Substituents on Acid Strength:
- Acetic Acid: The conjugate base (CH₃COO⁻) is stabilized by resonance but is affected by the electron-donating nature of the methyl group (CH₃), which decreases the stability of the negative charge.
- Fluoroacetic Acid: The fluorine atom is highly electronegative and acts as an electron-withdrawing group, stabilizing the conjugate base (CHF₂COO⁻) by dispersing the negative charge. This makes fluoroacetic acid stronger than acetic acid.
- 4-Nitrobenzoic Acid: The nitro group (NO₂) is a strong electron-withdrawing group, which stabilizes the conjugate base (C₆H₄(NO₂)COO⁻) significantly, making this acid stronger than both acetic acid and fluoroacetic acid.
- 4-Methylbenzoic Acid: The methyl group (CH₃) is an electron-donating group, which destabilizes the conjugate base (C₆H₄(CH₃)COO⁻), making this acid the weakest among the four.
3. Determine the Order of Acid Strength:
- Based on the stability of the conjugate bases, we can conclude:
- 4-Nitrobenzoic Acid > Fluoroacetic Acid > Acetic Acid > 4-Methylbenzoic Acid
Final Answer:
The correct order of acid strength is:
4-Nitrobenzoic Acid > Fluoroacetic Acid > Acetic Acid > 4-Methylbenzoic Acid
- Acetic Acid (CH₃COOH): Upon losing H⁺, it forms the conjugate base CH₃COO⁻.
- Fluoroacetic Acid (CHF₂COOH): Upon losing H⁺, it forms the conjugate base CHF₂COO⁻.
- 4-Nitrobenzoic Acid (C₆H₄(NO₂)COOH): Upon losing H⁺, it forms the conjugate base C₆H₄(NO₂)COO⁻.
- 4-Methylbenzoic Acid (C₆H₄(CH₃)COOH): Upon losing H⁺, it forms the conjugate base C₆H₄(CH₃)COO⁻.
- Acetic Acid: The conjugate base (CH₃COO⁻) is stabilized by resonance but is affected by the electron-donating nature of the methyl group (CH₃), which decreases the stability of the negative charge.
- Fluoroacetic Acid: The fluorine atom is highly electronegative and acts as an electron-withdrawing group, stabilizing the conjugate base (CHF₂COO⁻) by dispersing the negative charge. This makes fluoroacetic acid stronger than acetic acid.
- 4-Nitrobenzoic Acid: The nitro group (NO₂) is a strong electron-withdrawing group, which stabilizes the conjugate base (C₆H₄(NO₂)COO⁻) significantly, making this acid stronger than both acetic acid and fluoroacetic acid.
- 4-Methylbenzoic Acid: The methyl group (CH₃) is an electron-donating group, which destabilizes the conjugate base (C₆H₄(CH₃)COO⁻), making this acid the weakest among the four.
- Based on the stability of the conjugate bases, we can conclude:
- 4-Nitrobenzoic Acid > Fluoroacetic Acid > Acetic Acid > 4-Methylbenzoic Acid
The correct order of acid strength is:
4-Nitrobenzoic Acid > Fluoroacetic Acid > Acetic Acid > 4-Methylbenzoic Acid
Similar Questions
Knowledge Check
Correct order of acidic strength
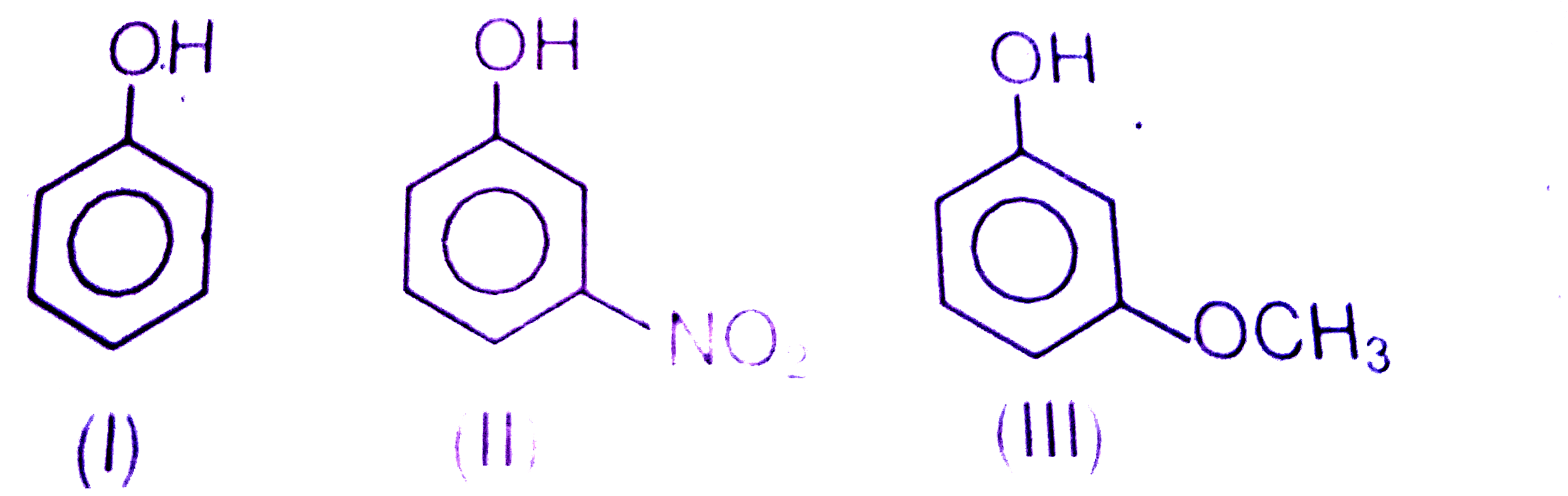 AI>II>IIIBII>III>ICI>III>IIDII>II>I
AI>II>IIIBII>III>ICI>III>IIDII>II>IThe correct order of acid strength is
AHCIO4<HCIO3<HCIO2<HCIOBHCIO2<HCIO3<HCIO4<HCIOCHCIO4<HCIO<HCIO2<HCIO3DHCIO<HCIO2<HCIO3<HCIO4The correct order of acidic strength is
AK2O>CaO>MgOBCO2>N2O5>SO3CNa2O>MgO>Al2O3DCl2O7>SO2>P4O10
Correct order of acidic strength
(I)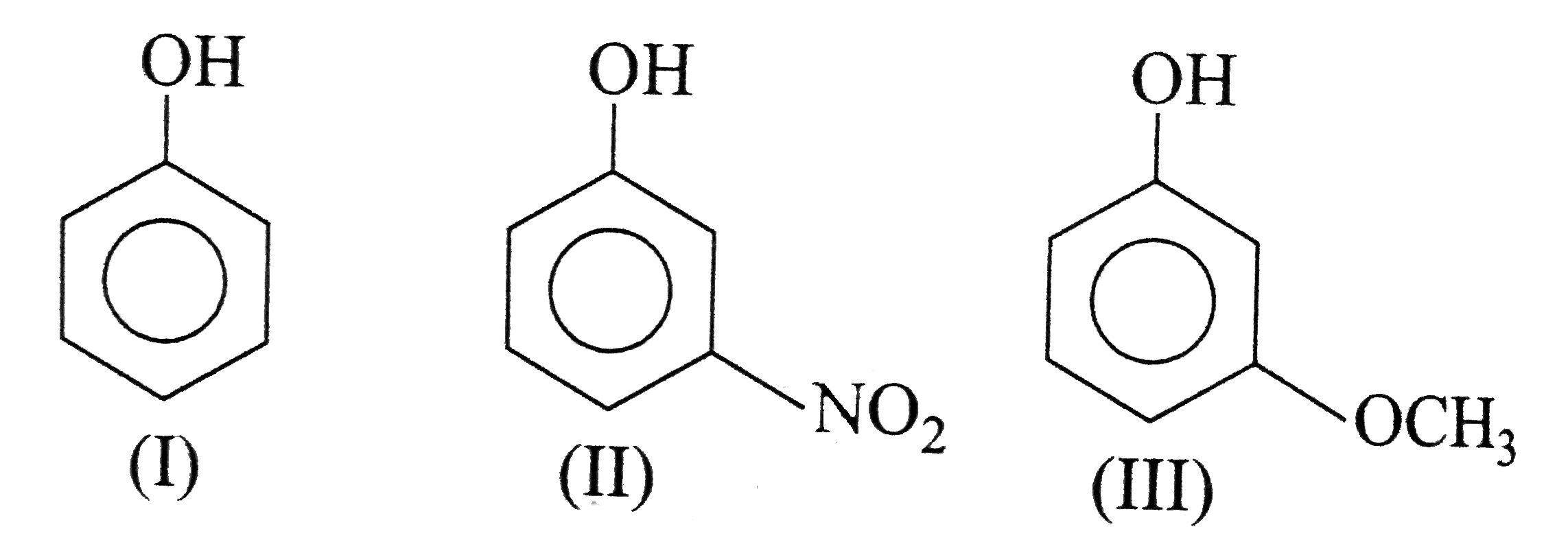 (II)
(II)  (III)
(III) 
View SolutionThe correct order of acidic strength of carboxylic acid is
View SolutionThe correct order of acid strength of following aromatic acids is : Benzoic acid (I), 4-Nitrobenzoic acid (II), 4-Dinitrobenzoic acid (III), 4-Methoxybenzoic acid (IV)
View SolutionThe correct order of acidic strength of carboxylic acid is
View SolutionThe correct order of acid strengths of benzoic acid (X) peroxybenzoic acid (Y) and p-nitrobenzoic acid (Z) is
View Solution
