The strongest base amongst the following is
Methanamine
N-Methylmethanamine
N-Methylaniline
N,N-Dimethylmethanamine
To determine the strongest base among the given compounds, we need to analyze the structures and the availability of lone pairs for donation. The compounds in question are:
1. Methanamine (CH3NH2)
2. N-methylmethanamine (CH3NHCH3)
3. N-methylaniline (C6H5NHCH3)
4. N,N-dimethylmethanamine (CH3N(CH3)2)
Step 1: Identify the lone pairs in each compound.
- Methanamine has one lone pair on the nitrogen atom.
- N-methylmethanamine has one lone pair on the nitrogen atom.
- N-methylaniline has one lone pair on the nitrogen atom, but it is partially delocalized into the aromatic ring.
- N,N-dimethylmethanamine has one lone pair on the nitrogen atom.
Hint: Look for the presence of lone pairs on the nitrogen atom in each compound.
Step 2: Analyze the effect of substituents on the nitrogen atom.
- In methanamine, the nitrogen is bonded to one alkyl group (methyl).
- In N-methylmethanamine, the nitrogen is bonded to two alkyl groups (one methyl and one hydrogen).
- In N-methylaniline, the nitrogen is bonded to one alkyl group and is also part of an aromatic system, which affects the availability of the lone pair due to resonance.
- In N,N-dimethylmethanamine, the nitrogen is bonded to two methyl groups, which increases the electron density on nitrogen.
Hint: Consider how alkyl groups (electron-donating) influence the availability of the lone pair on nitrogen.
Step 3: Consider the resonance effect in N-methylaniline.
- In N-methylaniline, the lone pair on nitrogen can participate in resonance with the benzene ring, making it less available for protonation.
- This reduces the basicity of N-methylaniline compared to the other amines.
Hint: Remember that resonance can stabilize a lone pair, making it less available for donation.
Step 4: Compare the basicity of the remaining amines.
- Methanamine and N-methylmethanamine both have lone pairs that are readily available for donation, but N,N-dimethylmethanamine has two alkyl groups which significantly increase the electron density on nitrogen, enhancing its ability to donate the lone pair.
- Therefore, N,N-dimethylmethanamine is expected to be the strongest base.
Hint: The more alkyl groups attached to nitrogen, the stronger the base due to increased electron density.
Final Answer:
The strongest base among the given compounds is N,N-dimethylmethanamine.
2. N-methylmethanamine (CH3NHCH3)
3. N-methylaniline (C6H5NHCH3)
4. N,N-dimethylmethanamine (CH3N(CH3)2)
- Methanamine has one lone pair on the nitrogen atom.
- N-methylmethanamine has one lone pair on the nitrogen atom.
- N-methylaniline has one lone pair on the nitrogen atom, but it is partially delocalized into the aromatic ring.
- N,N-dimethylmethanamine has one lone pair on the nitrogen atom.
- In methanamine, the nitrogen is bonded to one alkyl group (methyl).
- In N-methylmethanamine, the nitrogen is bonded to two alkyl groups (one methyl and one hydrogen).
- In N-methylaniline, the nitrogen is bonded to one alkyl group and is also part of an aromatic system, which affects the availability of the lone pair due to resonance.
- In N,N-dimethylmethanamine, the nitrogen is bonded to two methyl groups, which increases the electron density on nitrogen.
- In N-methylaniline, the lone pair on nitrogen can participate in resonance with the benzene ring, making it less available for protonation.
- This reduces the basicity of N-methylaniline compared to the other amines.
- Methanamine and N-methylmethanamine both have lone pairs that are readily available for donation, but N,N-dimethylmethanamine has two alkyl groups which significantly increase the electron density on nitrogen, enhancing its ability to donate the lone pair.
- Therefore, N,N-dimethylmethanamine is expected to be the strongest base.
The strongest base among the given compounds is N,N-dimethylmethanamine.
Similar Questions
Knowledge Check
The strongest base amongst the following in (In aqueous state):
ANH3BC2H5NH2C(C2H5)2ND(C2H5)3NThe strongest base among the following is
A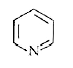 B
B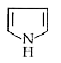 C
C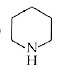 D
D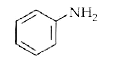
The strongest base among the following is
AC6H5NH2B(C6H5)2NHCC2H5NH2D(C2H5)2NH

