Write the reactions to bring about the following conversions.
.Aniline to Sulfanilic acid
To convert aniline to sulfanilic acid, we can follow these steps:
Step 1: Formation of Anilinium Hydrogen Sulfate
1. Reaction: Aniline reacts with concentrated sulfuric acid (H₂SO₄).
C6H5NH2+H2SO4→C6H5NH+3HSO−4
- Explanation: Aniline (C₆H₅NH₂) reacts with concentrated sulfuric acid to form anilinium hydrogen sulfate (C₆H₅NH₃⁺ HSO₄⁻).
Step 2: Conversion to Sulfanilic Acid
2. Reaction: Heating the anilinium hydrogen sulfate.
C6H5NH+3HSO−4Heat (453-473 K)−−−−−−−−−−→C6H5SO3NH2+H2O
- Explanation: Upon heating the anilinium hydrogen sulfate at a temperature between 453 to 473 K, sulfanilic acid (C₆H₅SO₃NH₂) is formed along with water.
Final Reaction
The overall reaction can be summarized as:
C6H5NH2+H2SO4Heat−−→C6H5SO3NH2+H2O
Summary
Thus, the conversion of aniline to sulfanilic acid involves the formation of anilinium hydrogen sulfate followed by heating to produce sulfanilic acid.
---
1. Reaction: Aniline reacts with concentrated sulfuric acid (H₂SO₄).
C6H5NH2+H2SO4→C6H5NH+3HSO−4
- Explanation: Aniline (C₆H₅NH₂) reacts with concentrated sulfuric acid to form anilinium hydrogen sulfate (C₆H₅NH₃⁺ HSO₄⁻).
2. Reaction: Heating the anilinium hydrogen sulfate.
C6H5NH+3HSO−4Heat (453-473 K)−−−−−−−−−−→C6H5SO3NH2+H2O
- Explanation: Upon heating the anilinium hydrogen sulfate at a temperature between 453 to 473 K, sulfanilic acid (C₆H₅SO₃NH₂) is formed along with water.
The overall reaction can be summarized as:
C6H5NH2+H2SO4Heat−−→C6H5SO3NH2+H2O
Thus, the conversion of aniline to sulfanilic acid involves the formation of anilinium hydrogen sulfate followed by heating to produce sulfanilic acid.
Similar Questions
Knowledge Check
Find the reagent used to bring about following conversions.
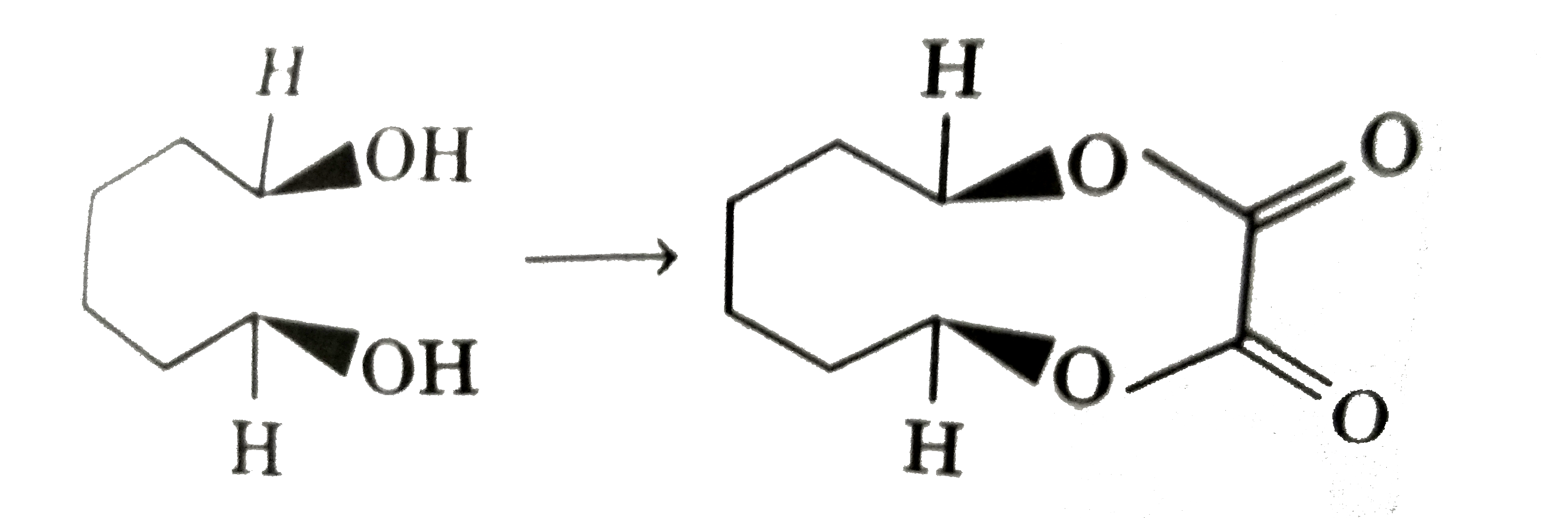 ACIOC−CH2−CH2COCIBCH3COOCOCH3CCH3COCIDCIOC−COCI
ACIOC−CH2−CH2COCIBCH3COOCOCH3CCH3COCIDCIOC−COCIWhich reagent brings about the following conversion ?
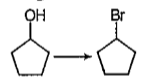 ABr2,Cl4BBr2,H2OCNaBrDPBr_3
ABr2,Cl4BBr2,H2OCNaBrDPBr_3Which reagent is used to bring about the following conversion?
 AKMnO4/H2OBH2O/H+CHydrogenation oxidation reactionDOSO4
AKMnO4/H2OBH2O/H+CHydrogenation oxidation reactionDOSO4
Write the reactions to bring about the following conversions.
Methyl chloride to Ethanamine
View SolutionHow will you bring about the following conversions ?
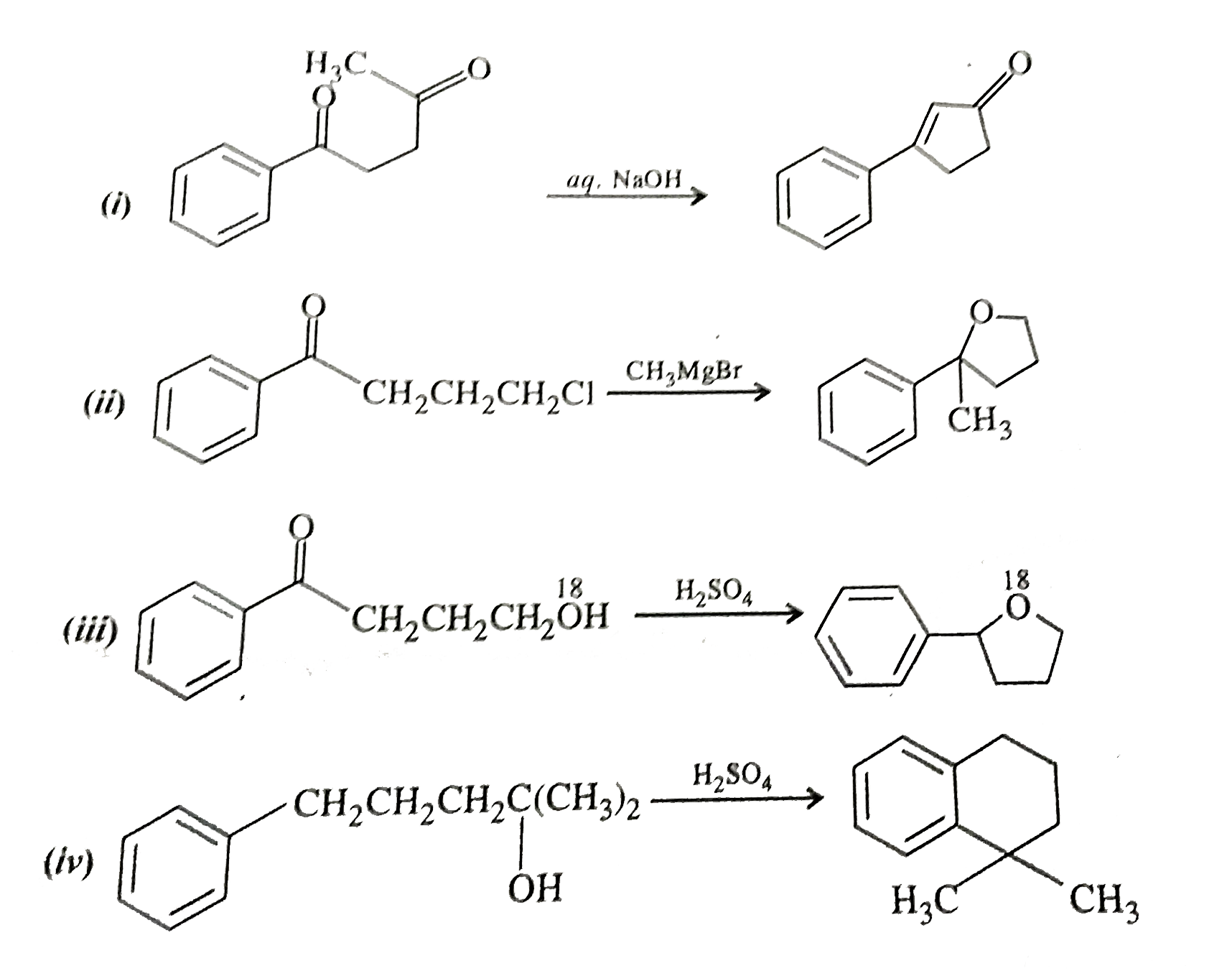
View SolutionHow will you bring out the following conversion?

View SolutionHow will you bring out the following conversion?
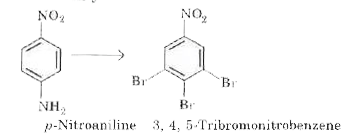
View SolutionDo the following conversions: Aniline to benzoic acid
View Solution
