Between hydrogen and helium which one has higher value of Henry's law of gas constant ?Solution in Odia
The correct Answer is:Helium.
Step by step video & text solution for Between hydrogen and helium which one has higher value of Henry's law of gas constant ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
Similar Questions
Tungsten crystallises in body centred cubic crystal. If the edge of the unit cell is 316.5 pm, what is the radius of tungsten atom?
View SolutionNegative catalyst is one :
View SolutionMolar solubility of helium, nitrogen and oxygen are plotted against partial pressure of the gas at constant temperature.
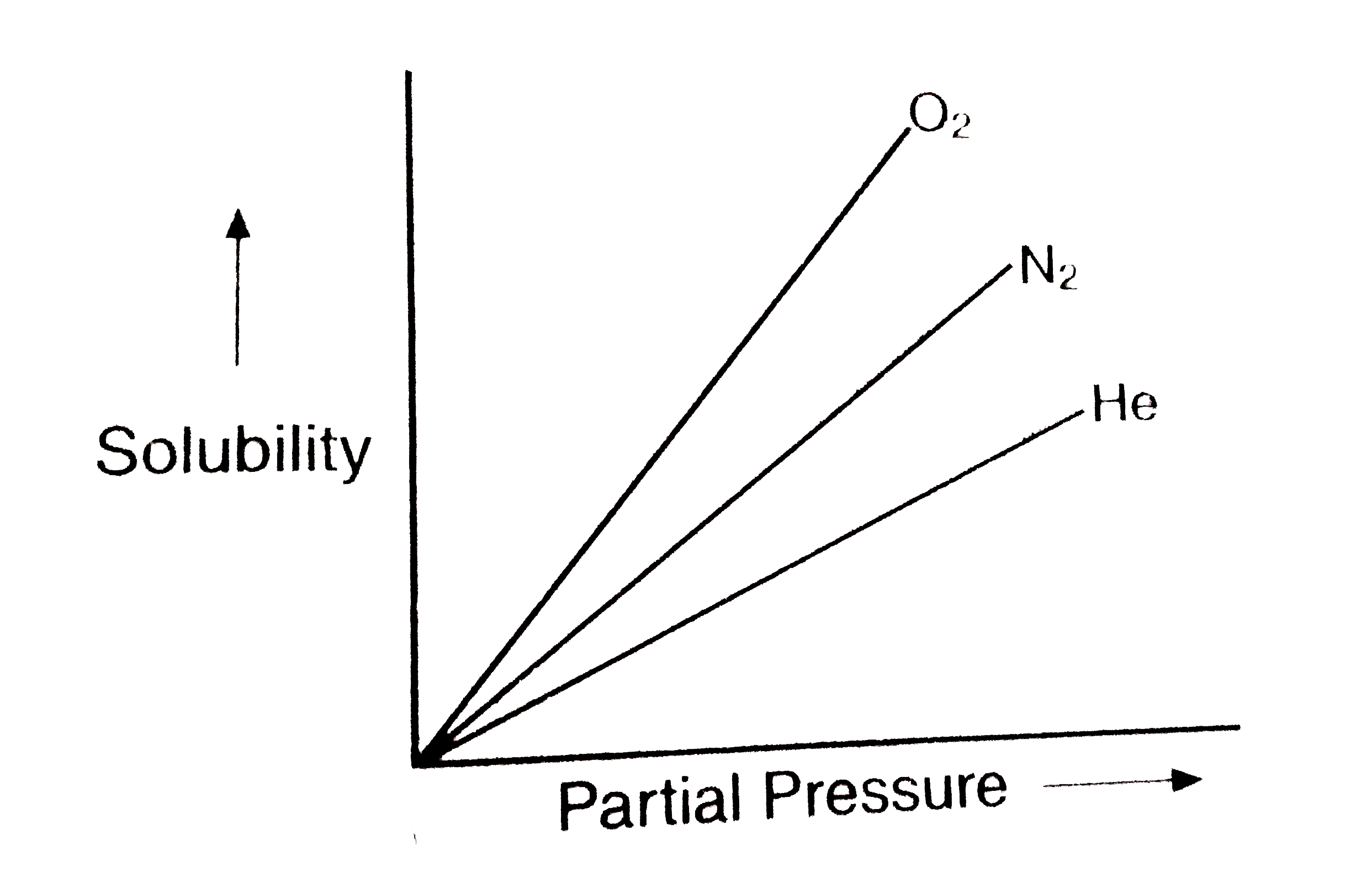
Henry's law constant for these gases will lie in following sequences?
View SolutionHow is Henry's Law constant related to the solubilitt of a gas in a solvent ?
View SolutionState Henry's Law for the solubility of a gas in liquid. Explain the singinficance of Henry's Law constant (KH. At the same temperature, hydrogen is more soluble in water than helium. Which of them has higher KH value and why ?
View Solutionरक्त में घुली-कौन-सी गैस पर हेनरी का नियम लागू नहीं होगा?
View SolutionWhich one of the following gases has the lowest value of Henry's law constant?
View SolutionWhich one of the following gases has the lowest value of Henry law constant ?
View SolutionAt given temperature and pressure nitrogenn gas is more soluble I water than helium gas. Which one of them has higher KH value.
View SolutionAt a given temperature and pressure nitrogen gas is more soluble in water than Helium gas. Which one of them has higher value of K_n ?
View SolutionWhich one of the following gases has the lowest value of Henry's law constant ?
View SolutionState Henry's law for slubility of a gas in a liquid. Explain the significance of Henry's law constnat (K_(H)). At the same temperatue, hydrogen is more soluble in water than helium. Which of them will have a higer value of K_(H) and Why?
View SolutionWhich one of the following gases has the lowest value of Henry's Law constant.
View SolutionWhich one of the following gases has the lowest value of Henry law constant ?
View SolutionAt a given temperature and pressure nitrogen gas is more soluble in water than helium gas. Which one of them has higher value of KH ?
View SolutionAt a given temperature and pressure nitrogen gas is more soluble in water than helium gas. Which one of them has higher value of KH ?
View Solution
