The number of bonds between carbon and oxygen atoms in major product of the following reaction is________.
CH3OH∣CHCH2CH2NH2ethyl formate (1 equiv)−−−−−−−−−−−−−→triethylam in e
The correct Answer is:c
To solve the question regarding the number of bonds between carbon and oxygen atoms in the major product of the given reaction, we will follow these steps:
1. Identify the Reactants: The reactants are 3-aminobutane (CH₃CH(CH₂)CH₂NH₂) and ethyl formate (HCOOC₂H₅).
2. Determine the Reaction Mechanism: In this reaction, the carbonyl carbon of ethyl formate will be attacked by the nitrogen atom of the amine (3-aminobutane) because nitrogen is less electronegative than oxygen and has a lone pair available for donation.
3. Formation of the Intermediate: When nitrogen attacks the carbonyl carbon, the carbonyl double bond (C=O) breaks. This results in the formation of a tetrahedral intermediate where the nitrogen is bonded to the carbon, and the oxygen carries a negative charge (O⁻).
4. Rearrangement and Elimination: The tetrahedral intermediate will rearrange, and the negatively charged oxygen will facilitate the departure of the ethyl group (C₂H₅O⁻), resulting in the formation of a new carbon-nitrogen bond.
5. Final Product Structure: After the rearrangement, the major product is a compound where the nitrogen is bonded to the carbon chain, and the carbonyl carbon is now bonded to a hydroxyl group (OH) and a carbonyl oxygen (C=O).
6. Count the Bonds: In the final product, we have:
- One carbon-oxygen double bond (C=O) which is a pi bond.
- One carbon-oxygen single bond (C-O) which is a sigma bond.
- Another carbon-oxygen single bond (C-O) from the hydroxyl group (OH).
Therefore, the total number of bonds between carbon and oxygen in the product is:
- 1 sigma bond (from C-O in OH)
- 1 sigma bond (from C-O in C=O)
- 1 pi bond (from C=O)
This gives us a total of 3 bonds (2 sigma bonds and 1 pi bond) between carbon and oxygen.
Final Answer:
The number of bonds between carbon and oxygen atoms in the major product of the reaction is 3.
- One carbon-oxygen double bond (C=O) which is a pi bond.
- One carbon-oxygen single bond (C-O) which is a sigma bond.
- Another carbon-oxygen single bond (C-O) from the hydroxyl group (OH).
- 1 sigma bond (from C-O in OH)
- 1 sigma bond (from C-O in C=O)
- 1 pi bond (from C=O)
The number of bonds between carbon and oxygen atoms in the major product of the reaction is 3.
Topper's Solved these Questions
- JEE MAIN REVISION TEST -14 Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST -14 Exercise:CHEMISTRY (SECTON 2)
Explore 5 Videos
- JEE MAIN REVISION TEST - 7|JEE - 2020Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 7|JEE - 2020Exercise:Chemistry (Section - 2) Numercial Value type
Explore 5 Videos
- JEE MAIN REVISION TEST -17 (2020)Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST -17 (2020)Exercise:CHEMISTRY
Explore 24 Videos
Similar Questions
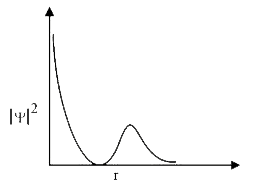
The graph for an orbital between |φ|2 and r(radial distance) is shown below. The sum of principle quantum number, azimuthal, quantum number and magnetic quantum number of this orbital is ____________.
View Solution2.44 g 'w' g of the benzoic acid dissolved in 30 g of benzene shows depression in freezing equal to 2K. If the percentage association of the acid to form polymer (C6H5COOH)n in the solution is 80, then find numerical value of n. (Given that Kf=5Kkgmol−1, Molar mass of benzoic acid =122gmol−1)
View SolutionThe gas phase reaction PCl5(g)⇔PCl3(g)+Cl2(g) is an endothermic reaction. The formation of PCl5 in equilibrium mixture of PCl5(g),PCl3(g)andCl2(g) can be decreased by :
View SolutionThe major product formed in the reaction
CH3−CH3∣C∣OH−CH=CH2+HBr→
is
View SolutionCH3−CH3∣C∣OH−CH3∣C∣NH2−CH3HNO2−−−→Δ?
View SolutionCH3−CH2CH3∣CHCH=CHCH3H3O+−−−→
Major product of the reaction
View SolutionThe major product of the following reaction is CH_(3)-overset(CH_(3))overset("| ")underset(OH)underset("| ")("C ")-underset("OH ")underset("| ")("CH"_(2)overset(H_(2)SO_(4))rarr
View SolutionThe major product of the following reaction is :
CH3C∣BrHCH2C∣BrHCH2CH3KOH,CH3OH−−−−−−−−−−−→heat
View SolutionThe major product formed in the reaction is:
CH3−CH3∣C∣H−CH2BrCH3OӨ−−−−→CH3OH
View SolutionThe IUPAC nameof CH3−Cl∣C∣OH−CH2−CH=CH−CH3 is :
View SolutionThe major product of the following reaction is:
CH3−CH3∣C∣H−CHCH3∣BrCH3OH−−−−→
View SolutionThe number of bonds between carbon and oxygen atoms in major product of the following reaction is________. CH_(3)overset(OH)overset(|)(CHCH_(2)CH_(2)NH_(2)) overset("ethyl formate (1 equiv)")underset("triethylam in e")to
View SolutionThe major product (A) formed in the following reaction is
CH3−CH3∣CH−CH2−CH3−CH−CH3∣N∣CH3−⊕CH2−CH3OH−AΔ
View SolutionThe major product in the reaction, CH_(3)CH_(2)NH_(2)+Cl-overset(O)overset(||)C-CH_(3) is
View SolutionGive IUPAC names of the following compounds:
(a) CH3−C∣OHH−CH2−C∣OHH−C∣C2H5H−CH2CH3 (b) CH3−C∣OHH−C∣OHH−CH3
(c)CH3C∣OHHCH2C∣CH3HCH2CH2CH2CH3 (d) CH3C∣CH3H−C∣OHH−CH3∣C∣CH3−CH3
(e) CH3C∣OHHC∣OHHCH2OH (f) C6H5CH2OH
(g) CH3CH2CHCH4OH (h) CH3CH2CH2OH∣C∣C6H5CH2CH3
(i) CH3OH∣CHCH2OH∣CHC∣CH3H−CH3 (j)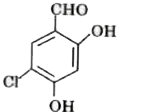
View SolutionThe major product of the following reaction is :
CH3OH∣CHCH2CH2NH2ethyl formate(1 equiv.)−−−−−−−−−−−−−→triethylamine
View Solution
