Borax Bead Test is carried out when the original mixture is coloured. It is done with the help of a cleam platinum wire on which a small loop is made at the end. When borax is heated on platinum wire loop transparent glass like bead is obtained. The hot bead is brought in contact with salt till it reacts with fused borax and colour is imparted to the bead. bead colour is noted.
Colour of the beadIon1. Blue greenCu2+2. Yellow Fe3+3. GreenCr3+4. VioletMn2+5. Dark blueCo2+6. BrownNi2+
Q. Glassy bead of :
sodium tetraborate
sodium metaborate
sodium metabarate and boric anhydride
sodium tetraborate and boric anhydride
The correct Answer is:C
To solve the question regarding the composition of the glassy bead formed during the borax bead test, we can follow these steps:
1. Understanding the Borax Bead Test:
- The borax bead test is a qualitative analysis technique used to identify certain metal ions based on the color of the bead formed when borax is heated and then mixed with the metal salt.
2. Formation of the Bead:
- When borax (sodium tetraborate) is heated on a platinum wire, it melts and forms a transparent glass-like bead.
3. Composition of the Bead:
- The glassy bead primarily consists of sodium metaborate (NaBO2) and boric anhydride (B2O3). These compounds are formed when borax is heated.
4. Identifying the Components:
- Sodium metaborate (NaBO2) is formed from the sodium ions and borate ions present in the borax.
- Boric anhydride (B2O3) is a result of the dehydration of boric acid, which is also related to the borax.
5. Conclusion:
- Therefore, the composition of the glassy bead can be expressed as a combination of sodium metaborate and boric anhydride.
6. Final Answer:
- The formula for the glassy bead is NaBO2 (sodium metaborate) and B2O3 (boric anhydride).
- The borax bead test is a qualitative analysis technique used to identify certain metal ions based on the color of the bead formed when borax is heated and then mixed with the metal salt.
- When borax (sodium tetraborate) is heated on a platinum wire, it melts and forms a transparent glass-like bead.
- The glassy bead primarily consists of sodium metaborate (NaBO2) and boric anhydride (B2O3). These compounds are formed when borax is heated.
- Sodium metaborate (NaBO2) is formed from the sodium ions and borate ions present in the borax.
- Boric anhydride (B2O3) is a result of the dehydration of boric acid, which is also related to the borax.
- Therefore, the composition of the glassy bead can be expressed as a combination of sodium metaborate and boric anhydride.
- The formula for the glassy bead is NaBO2 (sodium metaborate) and B2O3 (boric anhydride).
Topper's Solved these Questions
- JEE MAIN REVISION TEST - 1 | JEE - 2020 Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 1 | JEE - 2020 Exercise:CHEMISTRY ( SECTION 2)
Explore 5 Videos
- JEE MAIN REVISION TEST - 1 (2020)Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 1 (2020)Exercise:CHEMISTRY - SECTION 2
Explore 5 Videos
- JEE MAIN REVISION TEST - 10| JEE -2020Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 10| JEE -2020Exercise:CHEMISTRY (SECTION 2)
Explore 5 Videos
Similar Questions
The enthalpy changes at 298 K in successive breaking of O−H bonds of water, are
H2O(g)→H(g)+OH(g),ΔH=498kJmol−1
OH(g)→H(g)+O(g),ΔH=428kJmol−1
The bond energy of the O-H bond is
View SolutionA solution containing 62g ethylene glycol in 276g water is cooled to −10∘C. If Kf for water is 1.86kgmol−1, the amount of water (in moles) separated as ice is ________.
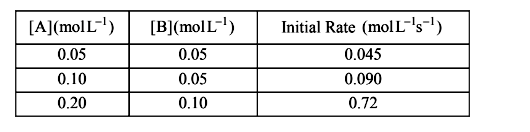
View SolutionFor the reaction 2A+B→C, the values of initial rate at different reactant concentrations are given in the table below. The over all order of reaction is __________.
View SolutionBorax when heated on platinum wire forms a glass like bead which is made up of :
View SolutionOn dipping a loop of platinum wire in borax paste and heating the wire subsequently in hot oxidising flame, produces a colourless, transparent, glass-like bead. The bead contains:
View SolutionWhen borax is heated in a platinum loop, the transparent bead formed contains
View SolutionWhich metal salt gives a violet coloured bead in the borax bead test ?
View Solution$ Borax bead test is applicable to coloured salts ! In borax bead test, coloured salts are decomposed to give coloured metal metaborates.
View SolutionBorax Bead Test is carried out when the original mixture is coloured. It is done with the help of a cleam platinum wire on which a small loop is made at the end. When borax is heated on platinum wire loop transparent glass like bead is obtained. The hot bead is brought in contact with salt till it reacts with fused borax and colour is imparted to the bead. bead colour is noted. {:("Colour of the bead","Ion"),(1." Blue green",Cu^(2+)),(2." Yellow ",Fe^(3+)),(3." Green",Cr^(3+)),(4." Violet",Mn^(2+)),(5." Dark blue",Co^(2+)),(6." Brown",Ni^(2+)):} Q. Glassy bead of :
View SolutionBorax Bead Test is carried out when the original mixture is coloured. It is done with the help of a cleam platinum wire on which a small loop is made at the end. When borax is heated on platinum wire loop transparent glass like bead is obtained. The hot bead is brought in contact with salt till it reacts with fused borax and colour is imparted to the bead. bead colour is noted.
Colour of the beadIon1. Blue greenCu2+2. Yellow Fe3+3. GreenCr3+4. VioletMn2+5. Dark blueCo2+6. BrownNi2+
Q. Blue bead can be of:
View SolutionBorax Bead Test is carried out when the original mixture is coloured. It is done with the help of a cleam platinum wire on which a small loop is made at the end. When borax is heated on platinum wire loop transparent glass like bead is obtained. The hot bead is brought in contact with salt till it reacts with fused borax and colour is imparted to the bead. bead colour is noted. {:("Colour of the bead","Ion"),(1." Blue green",Cu^(2+)),(2." Yellow ",Fe^(3+)),(3." Green",Cr^(3+)),(4." Violet",Mn^(2+)),(5." Dark blue",Co^(2+)),(6." Brown",Ni^(2+)):} Q. The flame used in Borax Bead Test is:
View SolutionBorax when heated on platinum wire forms a glass - like bead which is made up of :
View SolutionBorax when heated on platinum wire forms a glass like bead which is made up of
View SolutionA glassy bead formed by heating borax on a platinum wire loop is
View SolutionWhen borax is heated in a Bunsen burner flame with CaO on a loop of platinum wire , a yellow coloured Co(BO2)2 bead is formed.
View SolutionColour of the bead in borax bead test mainly due to the formation of
View Solution
