Which of the following compounds is a weaker acid as compared to benzoic acid?

The correct Answer is:C
To determine which compound is a weaker acid compared to benzoic acid, we need to analyze the effects of substituents on the benzoic acid structure.
1. Understanding Benzoic Acid:
- Benzoic acid (C6H5COOH) is a carboxylic acid where a carboxyl group (-COOH) is attached to a benzene ring. Its acidic strength is influenced by the substituents on the benzene ring.
2. Identifying the Effects of Substituents:
- Substituents can either be electron-withdrawing groups (EWGs) or electron-donating groups (EDGs).
- EWGs increase the acidity of carboxylic acids by stabilizing the negative charge on the conjugate base (the carboxylate ion).
- EDGs decrease the acidity by destabilizing the negative charge on the conjugate base.
3. Analyzing the Options:
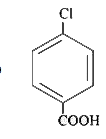
- Option A: Contains a chlorine substituent (Cl), which is an EWG. This would increase the acidity, making it stronger than benzoic acid.
- Option B: Contains a nitro group (NO2), which is also an EWG. This would also increase the acidity, making it stronger than benzoic acid.
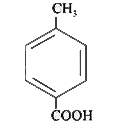
- Option C: Contains a methyl group (CH3), which is an EDG. This would decrease the acidity, making it weaker than benzoic acid.
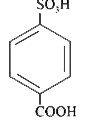
- Option D: Contains a sulfonic acid group (SO3H), which is an EWG. This would increase the acidity, making it stronger than benzoic acid.
4. Conclusion:
- Among the given options, the compound with the methyl group (Option C) is a weaker acid compared to benzoic acid.
Final Answer:
Option C (with CH3 group) is a weaker acid compared to benzoic acid.
- Benzoic acid (C6H5COOH) is a carboxylic acid where a carboxyl group (-COOH) is attached to a benzene ring. Its acidic strength is influenced by the substituents on the benzene ring.
- Substituents can either be electron-withdrawing groups (EWGs) or electron-donating groups (EDGs).
- EWGs increase the acidity of carboxylic acids by stabilizing the negative charge on the conjugate base (the carboxylate ion).
- EDGs decrease the acidity by destabilizing the negative charge on the conjugate base.
- Option A: Contains a chlorine substituent (Cl), which is an EWG. This would increase the acidity, making it stronger than benzoic acid.
- Option B: Contains a nitro group (NO2), which is also an EWG. This would also increase the acidity, making it stronger than benzoic acid.
- Option C: Contains a methyl group (CH3), which is an EDG. This would decrease the acidity, making it weaker than benzoic acid.
- Option D: Contains a sulfonic acid group (SO3H), which is an EWG. This would increase the acidity, making it stronger than benzoic acid.
- Among the given options, the compound with the methyl group (Option C) is a weaker acid compared to benzoic acid.
Option C (with CH3 group) is a weaker acid compared to benzoic acid.
Topper's Solved these Questions
- JEE MAIN REVISION TEST - 1 | JEE - 2020 Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 1 | JEE - 2020 Exercise:CHEMISTRY ( SECTION 2)
Explore 5 Videos
- JEE MAIN REVISION TEST - 1 (2020)Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 1 (2020)Exercise:CHEMISTRY - SECTION 2
Explore 5 Videos
- JEE MAIN REVISION TEST - 10| JEE -2020Book:VMC MODULES ENGLISHChapter:JEE MAIN REVISION TEST - 10| JEE -2020Exercise:CHEMISTRY (SECTION 2)
Explore 5 Videos
Similar Questions
Which of the following compound is tribasic acid?
View SolutionWhich of the following compounds is most acidic ?
View SolutionWhich of the following compounds is most acidic ?
View SolutionWhich of the following compounds is most acidic ?
View SolutionWhich of the following compound is tribasic acid?
View SolutionWhich of the following compounds are acidic?
View SolutionWhich of the following compound is most acidic?
View SolutionWhich of the following compound is most acidic?
View SolutionWhich of the following compounds is the most acidic ?
View SolutionExplain why:
Acetic acid is a weaker acid as com pared to formic acid.
View Solution
