The atomic radius of elements of which of the following series would be nearly the same
Na, K, Rb
Li, Be, B
F, Cl, Br
Fe, Co, Ni
The correct Answer is:D
To determine which series of elements has nearly the same atomic radius, we can analyze the options provided in the question step by step.
Step 1: Analyze Option A (Sodium, Potassium, Rubidium)
- Sodium (Na), Potassium (K), and Rubidium (Rb) are all alkali metals and belong to the same group (Group 1) in the periodic table.
- As we move down a group, the atomic radius increases due to the addition of electron shells.
- Therefore, the atomic radii will not be nearly the same: Rb > K > Na.
- Conclusion: This option is incorrect.
Step 2: Analyze Option B (Lithium, Beryllium, Boron)
- Lithium (Li), Beryllium (Be), and Boron (B) are in the same period (Period 2).
- As we move from left to right across a period, the atomic radius decreases due to increasing nuclear charge (more protons) which pulls the electrons closer to the nucleus.
- Thus, the atomic radii will be different: Li > Be > B.
- Conclusion: This option is also incorrect.
Step 3: Analyze Option C (Fluorine, Chlorine, Bromine)
- Fluorine (F), Chlorine (Cl), and Bromine (Br) are halogens and belong to the same group (Group 17).
- Similar to the alkali metals, as we move down the group, the atomic radius increases: Br > Cl > F.
- Therefore, the atomic radii will not be nearly the same.
- Conclusion: This option is incorrect as well.
Step 4: Analyze Option D (Iron, Cobalt, Nickel)
- Iron (Fe), Cobalt (Co), and Nickel (Ni) are transition metals and belong to the d-block of the periodic table.
- In the d-block, the atomic radius does not change significantly across the transition metals due to the balance between increasing nuclear charge and inter-electronic repulsion.
- The atomic radii of Fe, Co, and Ni are nearly the same because the effects of increasing nuclear charge are offset by the electron-electron repulsion in the d-orbitals.
- Conclusion: This option is correct as the atomic radii are nearly the same.
Final Answer
The atomic radius of elements in option D (Iron, Cobalt, Nickel) would be nearly the same.
---
- Sodium (Na), Potassium (K), and Rubidium (Rb) are all alkali metals and belong to the same group (Group 1) in the periodic table.
- As we move down a group, the atomic radius increases due to the addition of electron shells.
- Therefore, the atomic radii will not be nearly the same: Rb > K > Na.
- Conclusion: This option is incorrect.
- Lithium (Li), Beryllium (Be), and Boron (B) are in the same period (Period 2).
- As we move from left to right across a period, the atomic radius decreases due to increasing nuclear charge (more protons) which pulls the electrons closer to the nucleus.
- Thus, the atomic radii will be different: Li > Be > B.
- Conclusion: This option is also incorrect.
- Fluorine (F), Chlorine (Cl), and Bromine (Br) are halogens and belong to the same group (Group 17).
- Similar to the alkali metals, as we move down the group, the atomic radius increases: Br > Cl > F.
- Therefore, the atomic radii will not be nearly the same.
- Conclusion: This option is incorrect as well.
- Iron (Fe), Cobalt (Co), and Nickel (Ni) are transition metals and belong to the d-block of the periodic table.
- In the d-block, the atomic radius does not change significantly across the transition metals due to the balance between increasing nuclear charge and inter-electronic repulsion.
- The atomic radii of Fe, Co, and Ni are nearly the same because the effects of increasing nuclear charge are offset by the electron-electron repulsion in the d-orbitals.
- Conclusion: This option is correct as the atomic radii are nearly the same.
The atomic radius of elements in option D (Iron, Cobalt, Nickel) would be nearly the same.
Topper's Solved these Questions
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:VMC MODULES ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:LEVEL -2
Explore 48 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:VMC MODULES ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:LEVEL -2 Numerical Value Type for JEE Main
Explore 13 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:VMC MODULES ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:JEE ADVANCE (ARCHIVE)
Explore 30 Videos
- CHEMICAL THERMODYNAMICS Book:VMC MODULES ENGLISHChapter:CHEMICAL THERMODYNAMICS Exercise:IN - CHAPTER EXERCISE - L
Explore 10 Videos
- ELECTROCHEMISTRYBook:VMC MODULES ENGLISHChapter:ELECTROCHEMISTRYExercise:IMPECCABLE
Explore 50 Videos
Similar Questions
An atom has electronic configuration 2, 8, 7. (a) What is the atomic number of this element?
(b) To which of the following elements would it be chemically similar? (Atomic numbers are given in parentheses.)
N(7) F(9) P(15) Ar(18)
View SolutionWhich of the following elements would lose an electron easily ?
View Solution
Knowledge Check
Which of the following reactions would produce same product?
A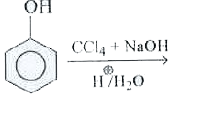 B
B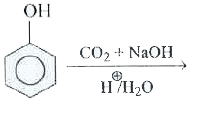 C
C D
D
Which of the following atom would likely form a cation.
View SolutionBecause of lanthnoid contraction, which of the following pairs of elements have nearly same atomic radii ? (Number in the parenthesis are atomic numbers)
View SolutionWhich of the following elements belongs to 4n- series?
View SolutionIf an element can have four values of spin quantum numbers and rest of the quantum no. are same, which of the following statement would be incorrect :-
View SolutionWhich of the following pairs have the same number of atoms?
View SolutionWhich of the following element belong to actinide series ?
View SolutionThere are 14 elements in actinoid series. Which of the following elements does not belong to this series?
View Solution
