All the products formed in the oxidation of NaBH4 by I2 are
B2H6 and NaI
B2H6,H2 and NaI
BI3 and NaH
NaBI4 and Hi
To determine the products formed in the oxidation of sodium borohydride (NaBH₄) by iodine (I₂), we can follow these steps:
Step 1: Identify the Reactants
The reactants in this reaction are sodium borohydride (NaBH₄) and iodine (I₂).
Step 2: Write the Chemical Reaction
The oxidation of sodium borohydride by iodine can be represented by the following chemical equation:
NaBH4+I2→NaI+B2H6+H2
Step 3: Analyze the Reaction
In this reaction:
- Sodium borohydride (NaBH₄) is oxidized, meaning it loses electrons.
- Iodine (I₂) is reduced, meaning it gains electrons.
- The products formed are sodium iodide (NaI), diborane (B₂H₆), and hydrogen gas (H₂).
Step 4: List the Products
From the balanced equation, the products formed are:
1. Sodium iodide (NaI)
2. Diborane (B₂H₆)
3. Hydrogen gas (H₂)
Step 5: Conclusion
Therefore, the complete list of products formed in the oxidation of NaBH₄ by I₂ is NaI, B₂H₆, and H₂.
Final Answer
The products formed are NaI, B₂H₆, and H₂.
---
The reactants in this reaction are sodium borohydride (NaBH₄) and iodine (I₂).
The oxidation of sodium borohydride by iodine can be represented by the following chemical equation:
NaBH4+I2→NaI+B2H6+H2
In this reaction:
- Sodium borohydride (NaBH₄) is oxidized, meaning it loses electrons.
- Iodine (I₂) is reduced, meaning it gains electrons.
- The products formed are sodium iodide (NaI), diborane (B₂H₆), and hydrogen gas (H₂).
From the balanced equation, the products formed are:
1. Sodium iodide (NaI)
2. Diborane (B₂H₆)
3. Hydrogen gas (H₂)
Therefore, the complete list of products formed in the oxidation of NaBH₄ by I₂ is NaI, B₂H₆, and H₂.
The products formed are NaI, B₂H₆, and H₂.
Topper's Solved these Questions
- p-BLOCK ELEMENTS-1BOOK - VMC MODULES ENGLISHCHAPTER - p-BLOCK ELEMENTS-1EXERCISE - LEVEL 2
73 Videos
- p-BLOCK ELEMENTS-1BOOK - VMC MODULES ENGLISHCHAPTER - p-BLOCK ELEMENTS-1EXERCISE - JEE Main Archive
34 Videos
- p-BLOCK ELEMENTS-1BOOK - VMC MODULES ENGLISHCHAPTER - p-BLOCK ELEMENTS-1EXERCISE - JEE Advanced Archive
48 Videos
- MOCK TEST 9BOOK - VMC MODULES ENGLISHCHAPTER - MOCK TEST 9EXERCISE - CHEMISTRY (SECTION 2)
5 Videos
- PERIODIC PROPERTIES OF ELEMENTSBOOK - VMC MODULES ENGLISHCHAPTER - PERIODIC PROPERTIES OF ELEMENTSEXERCISE - IMPECCABLE
50 Videos
Similar Questions
Name the by-products formed during the oxidation of food.
View SolutionGive the products formed by the oxidation of hot KMnO4.
a. Cyclobutene b. 1− Methyl cyclobutene
c. 1,2− Dimethyl cyclobutene
View SolutionWhat products are formed on thermal decomposition of (NH4)2BeF4?
View SolutionIdentify all the products formed in all the following reaction and indicate the major product
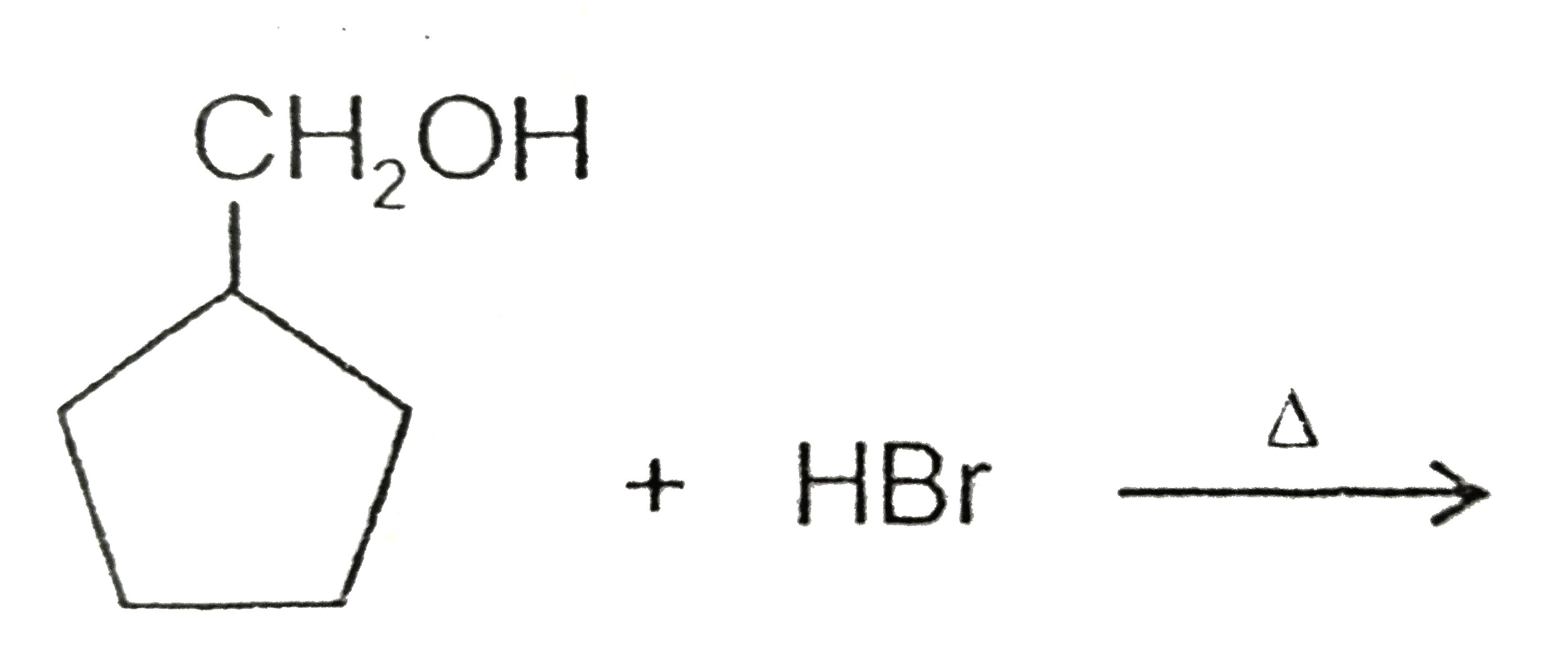
View SolutionGive the products formed on reduction with LAH and oxidation with H2O2, of the compound butan-2-one.
View SolutionOxidation of ketones involves carbon-carbon bond cleavage. Name the products formed on oxidation of 2, 5-dimethylhexan-3-one.
View SolutionWhat is the oxidation state of Mn in product formed by the oxidising action of KMnO4 inneutral medium ?
View SolutionThe major product formed in the reaction
CH3CH=CH−O∣∣C−CH3NaBH4−−−−−−−−→H2O,CH3OH is
View SolutionWhat is average oxidation state state of sulphur in product formed in given reaction?
Na2SO3+Na2S+I2→....+NaI
View SolutionWhat is average oxidation state state of sulphur in product formed in given reaction?
Na2SO3+Na2S+I2→....+NaI
View Solution

