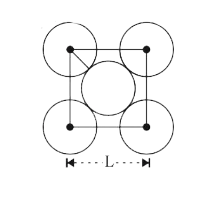
The packing efficiency of the two dimensional square unit cell shown below is:
A
0.3927
B
0.6802
C
0.7405
D
0.7854
Video Solution
Text Solution
√2L=4r=⇒rL=√24
Packing efficiency = 2πr2L2=2x(12√2)2=π4=78.5% .
|
Topper's Solved these Questions
- THE SOLID STATEBOOK - VMC MODULES ENGLISHCHAPTER - THE SOLID STATEEXERCISE - FUNDAMENTAL
50 Videos
- THE SOLID STATEBOOK - VMC MODULES ENGLISHCHAPTER - THE SOLID STATEEXERCISE - ENABLE
50 Videos
- THE SOLID STATEBOOK - VMC MODULES ENGLISHCHAPTER - THE SOLID STATEEXERCISE - JEE Main (Archive )
39 Videos
- SURFACE CHEMISTRYBOOK - VMC MODULES ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - PRACTICE EXERCISE
9 Videos
- THEORY OF SOLUTIONSBOOK - VMC MODULES ENGLISHCHAPTER - THEORY OF SOLUTIONSEXERCISE - JEE Advanced (Archive)
30 Videos
Similar Questions
Knowledge Check
the correct order of the packing effeciency in different types of unit cells is ……….. .
Afcc lt bcc lt simple cubicBfcc gt bcc gt simple cubicCfcc lt bcc gt simple cubicDbcc lt fcc gt simple cubic
The packing efficiency of the 2D square unit cell shown below is
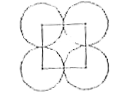
View SolutionThe efficiency of packing in simple cubic unit cell is
View Solutionthe correct order of the packing effeciency in different types of unit cells is ……….. .
View Solutionthe correct order of the packing effeciency in different types of unit cells is ……….. .
View SolutionWhat would be the efficiency of the heat engine diagramed as shown below?
_E01_064_Q01.png)
View SolutionAssertion: bcc and ccp have same packing efficiency
Reason: Both have same number of atoms per unit cell and same arrangement
View SolutionThe packing cells around the neurons in neural tissue are called
View Solution