A container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate
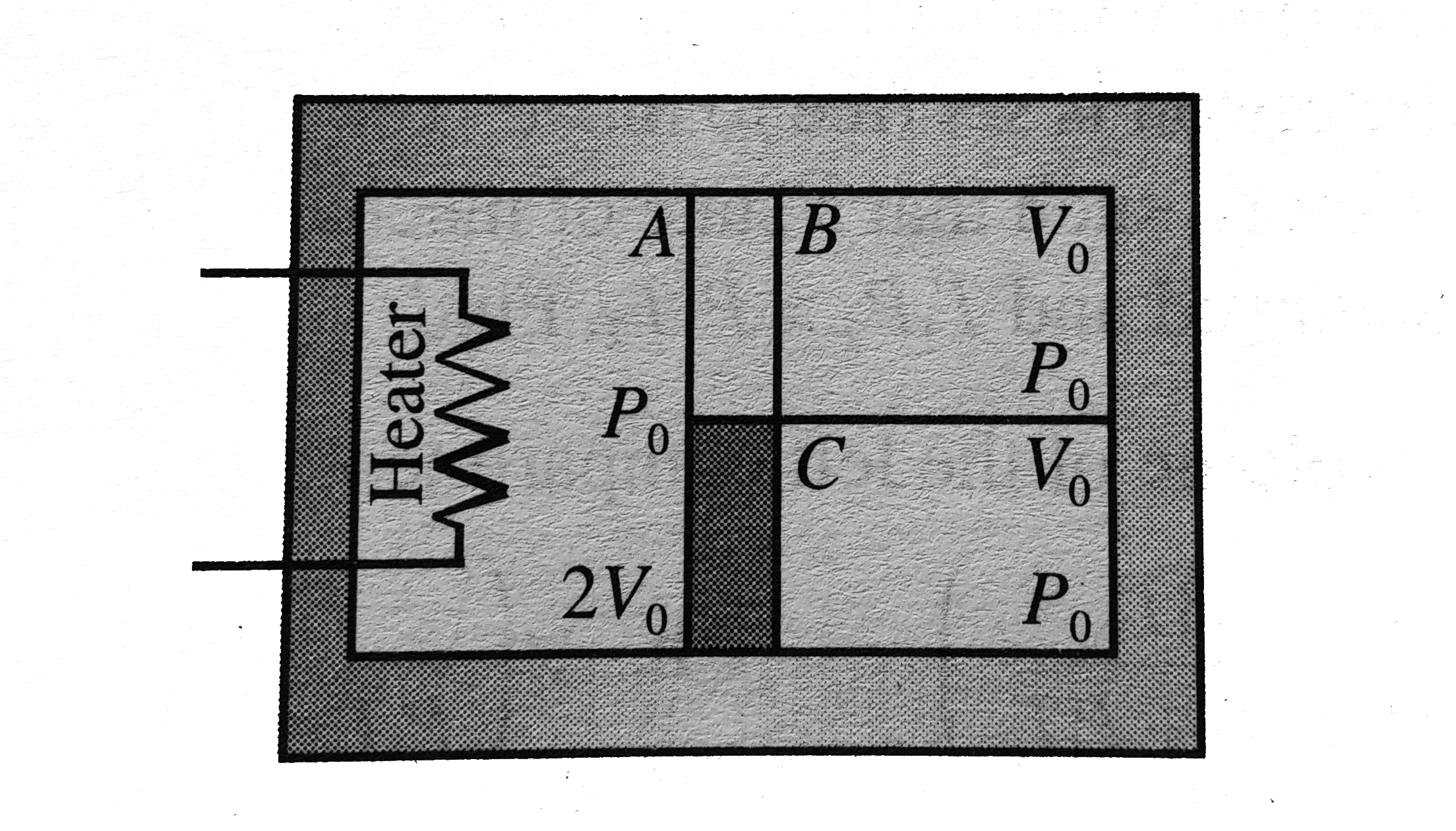
Find the temprature in part C
53P0V0
116P0V0
3156P0V0
3689P0V0
The correct Answer is:D
TA=TB=20527P0V0R
CV for gas =Rγ−1=2R
As ΔU=nCVΔT for any process,
ΔQ heaterΔUA+ΔUB+ΔUC=2×2×R(205278P0V0R)+2R(20527P0V0R−P0V0R)
+2R(53P0V0R−P0V0R)=3689P0V0
Topper's Solved these Questions
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:JEE MAIN (ARCHIVE )
Explore 81 Videos
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:JEE ADVANCED (ARCHIVE )
Explore 109 Videos
- GASEOUS STATE & THERMODYNAMICSBook:VMC MODULES ENGLISHChapter:GASEOUS STATE & THERMODYNAMICSExercise:Level - 1
Explore 75 Videos
- ENERGY & MOMENTUMBook:VMC MODULES ENGLISHChapter:ENERGY & MOMENTUMExercise:JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE
Explore 1 Video
- GRAVITATIONBook:VMC MODULES ENGLISHChapter:GRAVITATIONExercise:JEE Advance (Archive) TRUE/FALSE
Explore 1 Video
Similar Questions
A container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate
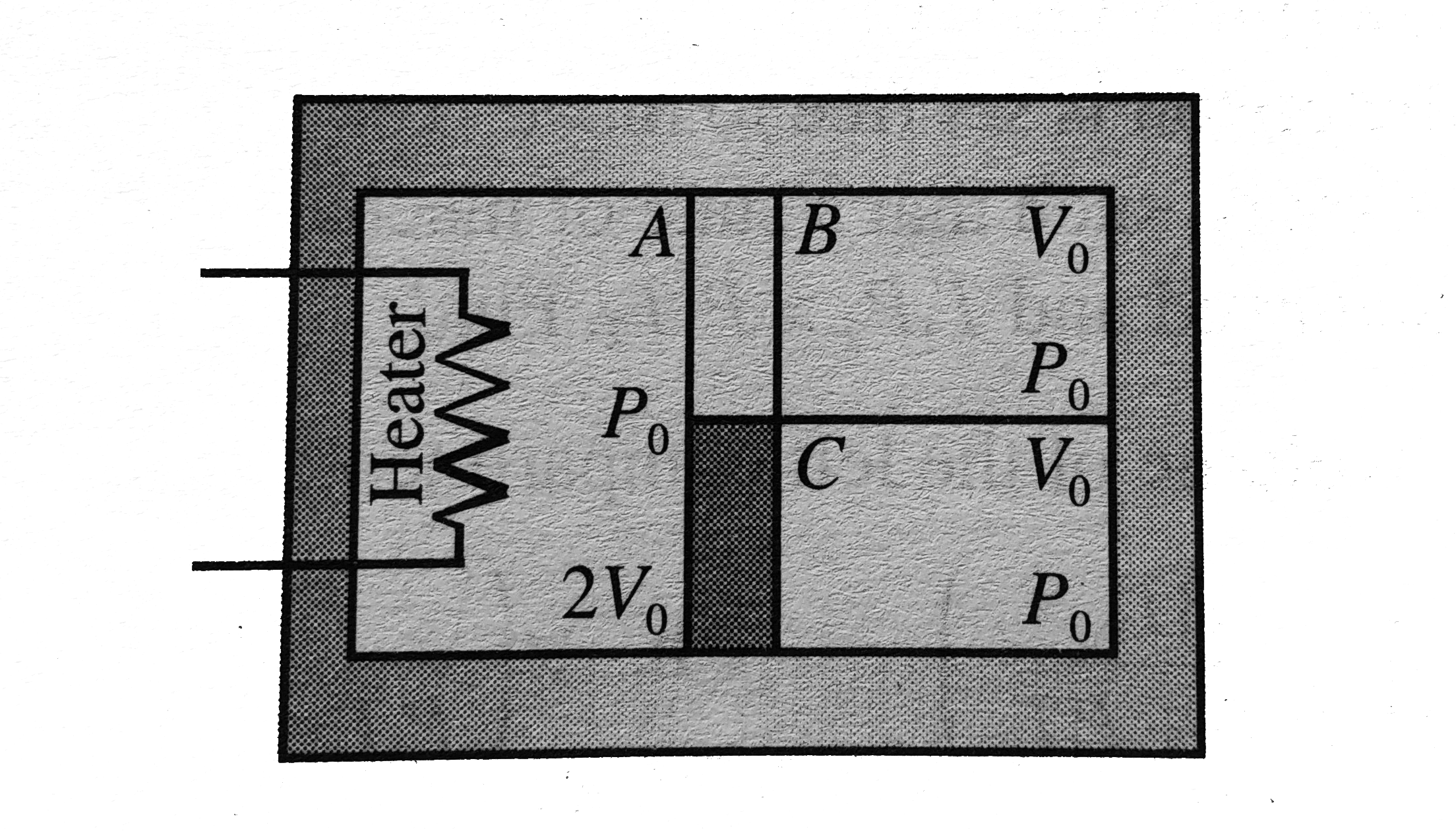
Final temperature in part C.
View SolutionA container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate

Final temperature in part C.
View SolutionA container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate

The heat supplied by the heater.
View SolutionA container of volume 4V0 made of a perfectly non− conducting material is divided into two equal parts by a fixed rigid wall whose lower half is non− conducting and upper half is purely conducting. The right side of the wall is divided into equal parts ( initially 0 by means of a massless non− conducting piston free to move as shown . SectionA constains 2mol of a gas while the section B and C contain 1mol each of the same gas (γ=15.) at pressure P0 . The heater in left part is switched on till the final pressure in section C becomes 125/27P0. Calculate

The heat supplied by the heater.
View SolutionWhich of the following is non-conducting?
View SolutionThe non conducting supporting cells of nervous tissue is
View SolutionA cylindrical vessel made of thermally insulating material is divided into two equal parts by an insulating and. Movable piston. Both parts contain ideal monoatomic gas . The gas in the left part is supplied heat such that the volume of right part becomes one-eight of its initial volume . Work done by the gas in the right part is
View SolutionThe conducting part of the respiratory system is
View SolutionThe solution of which of the following will be non - conducting ?
View SolutionThe figure shown an L-shaped rod rotating about its end O in a plane perpendicular to a magnetic field B. The part OA of rod is non conducting while AB is conducting the emf induced between A and B is

View Solution
