One mole of an ideal mono-atomic gas is taken round cyclic process ABC as shown in figure below. Calculate work done
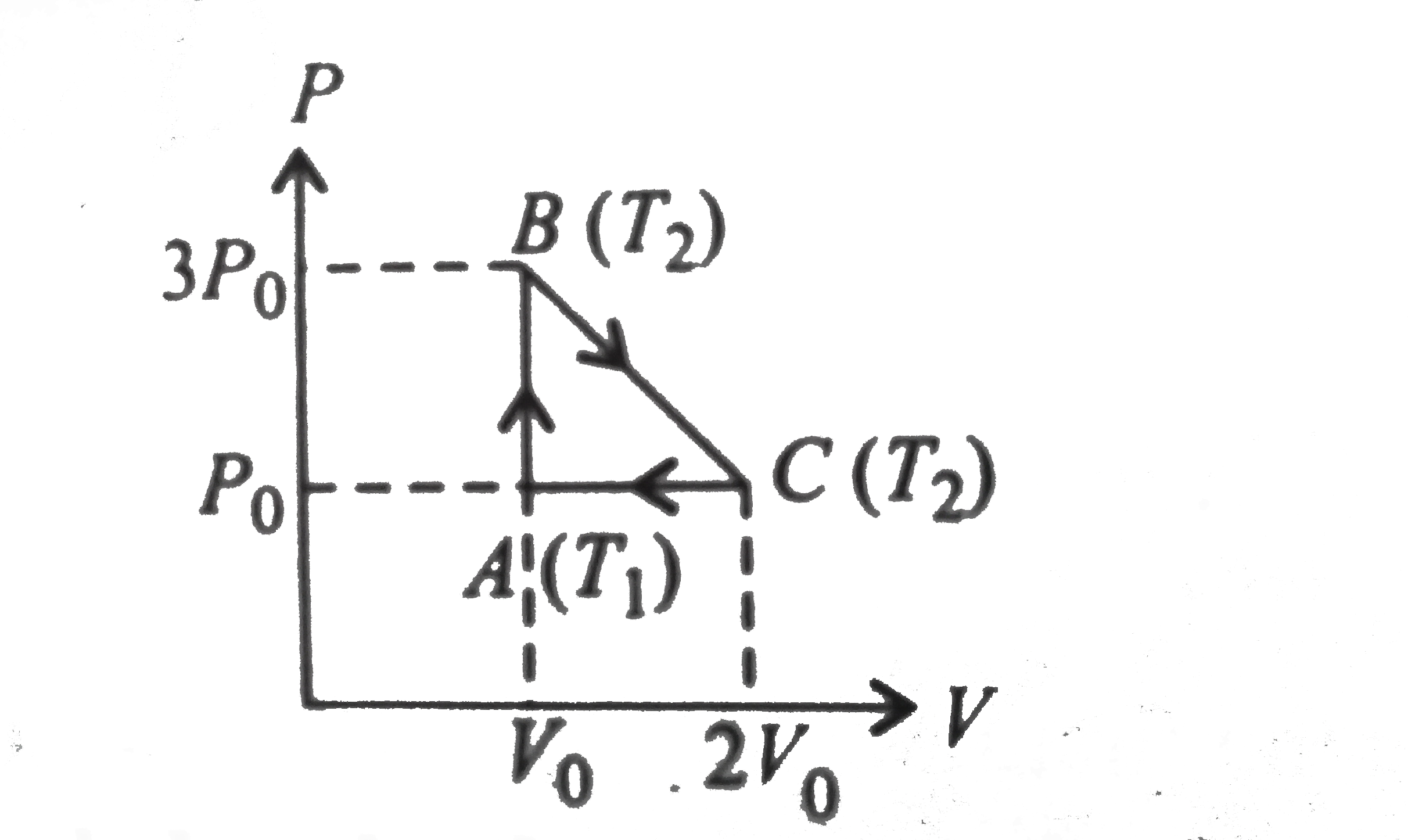
The correct Answer is:P0V02
Heat rejected in path CA (Process is isobaric)
∴ dQCA=CpdT=CP(Tf−Ti)
=Cp(pFVFR−PiViR)=CpR(PfVf−PioVi)
Substituting the values
dQCA=52(P0V0−2P0V0)=−52P0V0
Therefore, heat rejected in the process CA is 52p0V0
Topper's Solved these Questions
- GASEOUS STATE & THERMODYNAMICSBOOK - VMC MODULES ENGLISHCHAPTER - GASEOUS STATE & THERMODYNAMICSEXERCISE - JEE MAIN (ARCHIVE )
81 Videos
- ENERGY & MOMENTUMBOOK - VMC MODULES ENGLISHCHAPTER - ENERGY & MOMENTUMEXERCISE - JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE
1 Video
- GRAVITATIONBOOK - VMC MODULES ENGLISHCHAPTER - GRAVITATIONEXERCISE - JEE Advance (Archive) TRUE/FALSE
1 Video
Similar Questions
Explain why a Carnot engine cannot have 100% efficiency?
View SolutionTwo particles of mass 1 kg and 3 kg move towards each other under their mutual force of attraction. No other force acts on them. When the relative velocity of approach of the two particles is 2m/s, their centre of mass has a velocity of 0.5 m/s. When the relative velocity of approach becomes 3m/s, the velocity of the centre of mass is 0.75 m/s.
View SolutionIs it possible to put an artificial satellite in an orbit in such a way that it always remain visible directly over New Delhi ?
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABCD as shown in figure below. Calculate
View SolutionOne mole of ideal monoatomic gas is taken round the cyclic process as shown in figure. The heat rejected by the gas in the path is ………...and the heat absorbed by the gas in the path AB is ………...........
View SolutionAn ideal gas is taken around the cycle ABCA as shown below:
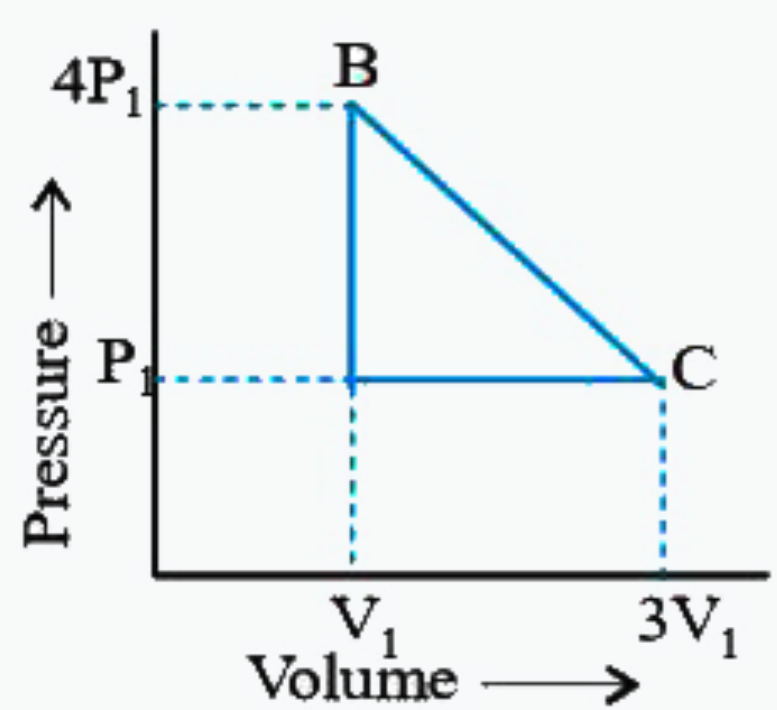
Work done in the cyclic process is
View SolutionAn ideal gas is taken in a cyclic process as shown in the figure. Calculate (a) work done by the gas. (b) work done on the gas (c ) Net work done in the process
View SolutionTwo moles of helium gas undergo a cyclic process as shown in figure. Assuming the gas to be ideal. The net work done by the gas is
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate
The work done by the gas
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate
The net heat absorbed by the gas in the path BC.
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate
The maximum temperature attained by the gas during the cycle
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure . Calculate the net heat absorbed by the gas in the path BC.
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure . Calculate
The maximum temperature attained by the gas during the cycle,
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABCD as shown in figure below. Calculate work done by the gas.
View SolutionOne mole of an ideal mono-atomic gas is taken round cyclic process ABC as shown in figure below. Calculate work done
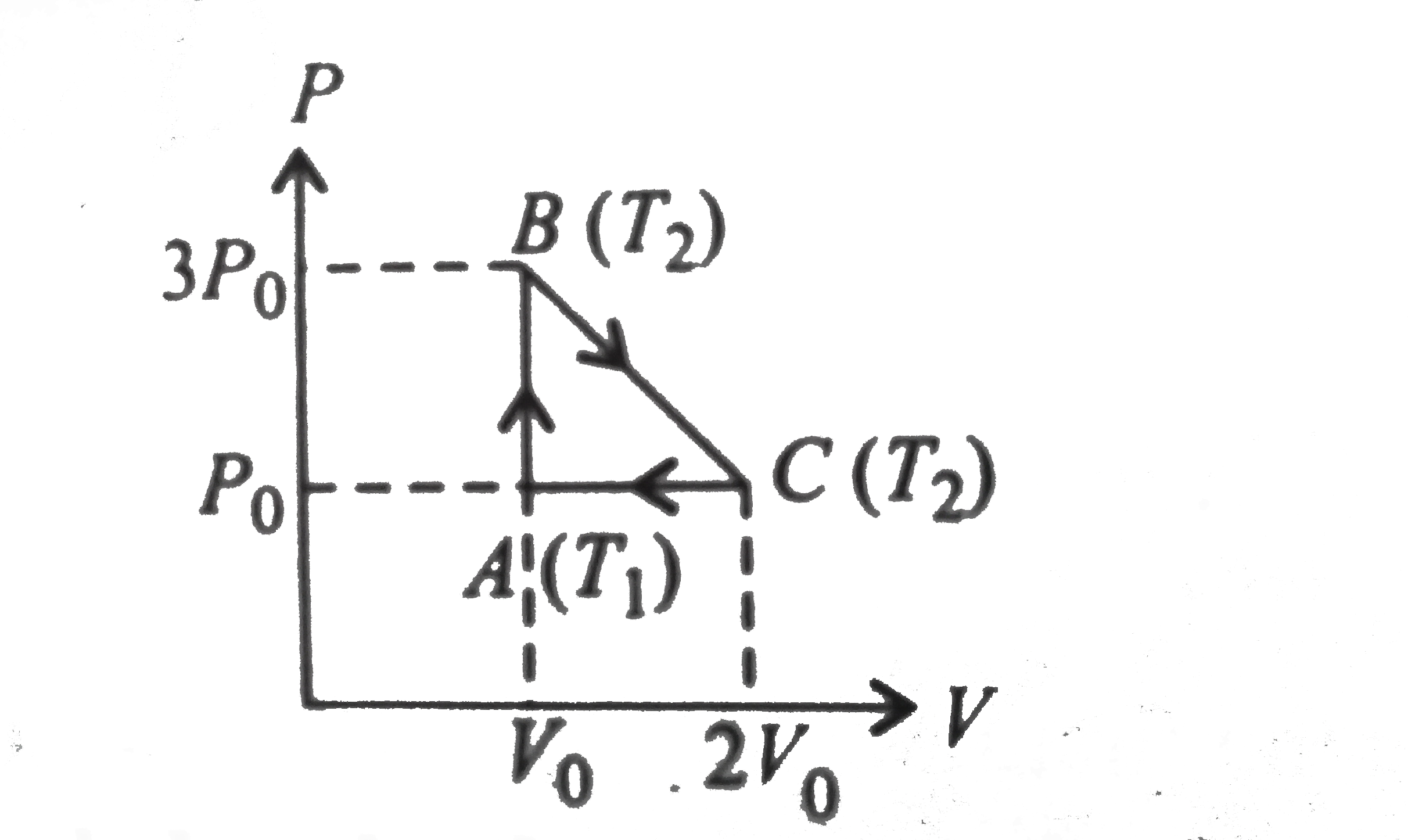
View Solution

