The following are some statements related to II group metals
I. The reaction with water increases from Be to Ba
II. The ionic nature of hydrides decreases from Be to Ba
III. The thermal stability of the peroxides increases with increase in cationic size
IV. The solubility of sulphates increases from Mg to Ba
The correct options are :
I & III
II & IV
I, II & III
I , III & IV
To solve the question regarding the statements related to Group II metals, we will analyze each statement one by one.
Step 1: Analyze Statement I
Statement I: The reaction with water increases from Be to Ba.
- Explanation:
- As we move down the group from Beryllium (Be) to Barium (Ba), the ionization energy decreases.
- Lower ionization energy means that it is easier for the metal to lose its outermost electron and react with water.
- Therefore, the reactivity with water indeed increases from Be to Ba.
Conclusion: Statement I is True.
Step 2: Analyze Statement II
Statement II: The ionic nature of hydrides decreases from Be to Ba.
- Explanation:
- According to Fajan's rules, the ionic character of a compound is directly proportional to the size of the cation.
- As we move down the group, the size of the cation increases (Be²⁺ < Mg²⁺ < Ca²⁺ < Sr²⁺ < Ba²⁺).
- Therefore, the ionic character of the hydrides actually increases as the cation size increases, not decreases.
Conclusion: Statement II is False.
Step 3: Analyze Statement III
Statement III: The thermal stability of the peroxides increases with increase in cationic size.
- Explanation:
- The thermal stability of peroxides is influenced by the size of the cation.
- Larger cations have lower charge density, which leads to lower polarization of the peroxide ion (O₂²⁻).
- As the size of the cation increases from Be to Ba, the thermal stability of the peroxides increases.
Conclusion: Statement III is True.
Step 4: Analyze Statement IV
Statement IV: The solubility of sulphates increases from Mg to Ba.
- Explanation:
- The solubility of sulphates in water generally increases down the group due to decreasing lattice energy and increasing hydration energy.
- Therefore, the solubility of sulphates does indeed increase from Mg to Ba.
Conclusion: Statement IV is True.
Final Conclusion:
The correct statements are I, III, and IV. Therefore, the correct options are I, III, and IV.
Statement I: The reaction with water increases from Be to Ba.
- As we move down the group from Beryllium (Be) to Barium (Ba), the ionization energy decreases.
- Lower ionization energy means that it is easier for the metal to lose its outermost electron and react with water.
- Therefore, the reactivity with water indeed increases from Be to Ba.
Statement II: The ionic nature of hydrides decreases from Be to Ba.
- According to Fajan's rules, the ionic character of a compound is directly proportional to the size of the cation.
- As we move down the group, the size of the cation increases (Be²⁺ < Mg²⁺ < Ca²⁺ < Sr²⁺ < Ba²⁺).
- Therefore, the ionic character of the hydrides actually increases as the cation size increases, not decreases.
Statement III: The thermal stability of the peroxides increases with increase in cationic size.
- The thermal stability of peroxides is influenced by the size of the cation.
- Larger cations have lower charge density, which leads to lower polarization of the peroxide ion (O₂²⁻).
- As the size of the cation increases from Be to Ba, the thermal stability of the peroxides increases.
Statement IV: The solubility of sulphates increases from Mg to Ba.
- The solubility of sulphates in water generally increases down the group due to decreasing lattice energy and increasing hydration energy.
- Therefore, the solubility of sulphates does indeed increase from Mg to Ba.
The correct statements are I, III, and IV. Therefore, the correct options are I, III, and IV.
Topper's Solved these Questions
- 13-14 HYDROGEN & S-BLOCK ELEMENTBOOK - VMC MODULES ENGLISHCHAPTER - 13-14 HYDROGEN & S-BLOCK ELEMENTEXERCISE - Level 2 (Numerical value type )
14 Videos
- 13-14 HYDROGEN & S-BLOCK ELEMENTBOOK - VMC MODULES ENGLISHCHAPTER - 13-14 HYDROGEN & S-BLOCK ELEMENTEXERCISE - JEE Main Archive
64 Videos
- 13-14 HYDROGEN & S-BLOCK ELEMENTBOOK - VMC MODULES ENGLISHCHAPTER - 13-14 HYDROGEN & S-BLOCK ELEMENTEXERCISE - Level 1
74 Videos
- ALCOHOLS, PHENOLS & ETHERS BOOK - VMC MODULES ENGLISHCHAPTER - ALCOHOLS, PHENOLS & ETHERS EXERCISE - IN-CHAPTER EXERCISE - L
10 Videos
Similar Questions
The following are some statements related to VA group hydrides
I) Reducing property increases from NH3 to BiH3
II) Tendency to donate lone pair decreases from NH3 to BiH3
III) Ease of replacing H with Cl decreases from NH3 to BiH3
IV) Ease of formation of hydrides decreases from NH3 to BiH3
The correct statement are
View SolutionThe thermal stability of alkaline earth metal carbonates increases from Be to Ba. This is because
View Solution
Knowledge Check
Assertion : Acidic character of group 16 hydrides increases from H2O to H2Te.
Reason : Thermal stablility of hydrides decreases down the group.AIf both assertion and reason are true and reason is the correct explanation of assertion.BIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assertion is true but reason is false.DIf both assertion and reason are false.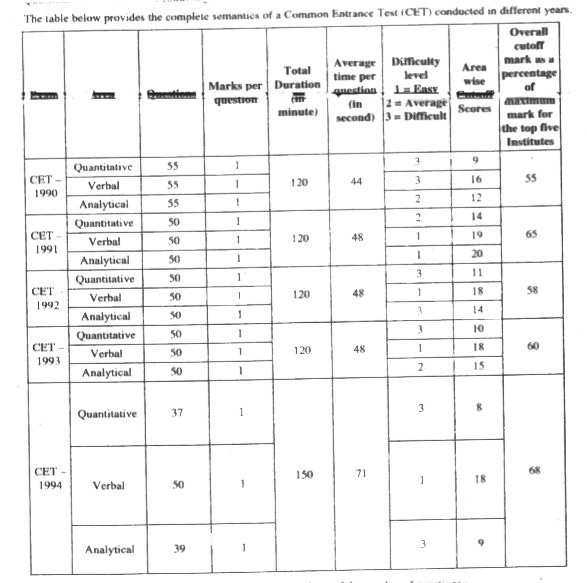
Which one of the following statements can be informed from the table ?
(I) As the Stress Factor increased, the cut off marks of the top five universities decreased
(II) As the difficulty Factor of the exam decreased, the cut off marks of the top five universities increased
(III) As the Difficulty Factor increased, the stress Factor increased.AI onlyBII onlyCIII onlyDI and II
Consider the following statements :
The mean free path of gas molecules
I. decreases with increase in concentration
II. increases with decrease in pressure at constant temperature
III. decreases with increase in molecular size
Which of the above statements are correct?
View SolutionIn which of the following options order of arrangement does not agree with the variatioin of property indicated against it?
Thinking process.
i) The ionic size increases as the positive charge on the cation decreases or the negative charge on the anion increases.
ii) First ionisation enthalpy increases from left to right in the periodic table.
iii) Electron gain enthalpy increases as the electronegativity of the atom increases.
iv) The metallic character increases as the size of the metal atom increases.
View SolutionWhich is correct statement ?
As the s-character of a hybrid orbital decreases
(I) The bond angle decreases (II) The bond strength increases
(III) The bond length increases (IV) Size of orbitals increases
View SolutionConsider the following statements
(I) Zr4+ and Hf4+ have almost same ionic radii
(II) Lanthanoids liberate H2 when treated with dilute acids.
(III) The ionic radii of trivalent lanthanoids steadily increase with increase in atomic number.
The correct statement(s) is/ are
View SolutionMetals have few electrons in their valence shell while non-metals generally have more electrons in their valence shell. Metallic character is closely related to atomic radius and ionisation enthalpy. Metallic character increases from top to bottom in a group and decreases from let to right in a period of periodic table. metallic character is inversely related to electronegativity of element.
Q. The electronegativity of the following elements increase in the order:
View SolutionWhich of the statement are in accordance with the observation of geographer Alexander von Humboldt ?
I. Within a region species, richness increases with increasing explored area but within a limit.
II. The relation between species richness and area is a rectangular hyperbola.
III. The richness of species decreases with the increasing explored area.
IV. There is no relation between the explored area and species richness .
View Solution

