In which one of the following conversions phosphorus pentachloride is used as a reagent?
H2C=CH2→CH3CH2Cl
HC ≡CH→CH2 = CHCl
CH3CH2 OH →CH3CH2 Cl
None of these
The correct Answer is:C
To solve the question regarding the use of phosphorus pentachloride (PCl5) as a reagent, we will analyze the given options and determine which conversion involves PCl5.
1. Understanding the Role of PCl5:
Phosphorus pentachloride is commonly used to convert alcohols into alkyl chlorides. It reacts with alcohols to replace the hydroxyl (-OH) group with a chlorine atom (-Cl).
2. Analyzing the Options:
We need to evaluate each option to see if it involves the conversion of an alcohol or similar compound using PCl5.
- Option A: This option does not involve an alcohol or a suitable substrate for PCl5. Therefore, PCl5 is not used here.
- Option B: Similar to option A, this option does not involve a reaction with an alcohol or a compound that would react with PCl5. Hence, PCl5 is not applicable.
- Option C: This option involves the reaction of ethanol (C2H5OH) with PCl5. The reaction proceeds as follows:
C2H5OH+PCl5→C2H5Cl+HCl+POCl3
In this reaction, the hydroxyl group (-OH) of ethanol is replaced by a chlorine atom (-Cl), resulting in the formation of ethyl chloride (C2H5Cl).
3. Conclusion:
After analyzing all the options, we find that phosphorus pentachloride is used as a reagent in Option C, where it reacts with ethanol to form ethyl chloride.
Final Answer:
Phosphorus pentachloride is used as a reagent in Option C.
Phosphorus pentachloride is commonly used to convert alcohols into alkyl chlorides. It reacts with alcohols to replace the hydroxyl (-OH) group with a chlorine atom (-Cl).
We need to evaluate each option to see if it involves the conversion of an alcohol or similar compound using PCl5.
- Option B: Similar to option A, this option does not involve a reaction with an alcohol or a compound that would react with PCl5. Hence, PCl5 is not applicable.
- Option C: This option involves the reaction of ethanol (C2H5OH) with PCl5. The reaction proceeds as follows:
C2H5OH+PCl5→C2H5Cl+HCl+POCl3
In this reaction, the hydroxyl group (-OH) of ethanol is replaced by a chlorine atom (-Cl), resulting in the formation of ethyl chloride (C2H5Cl).
After analyzing all the options, we find that phosphorus pentachloride is used as a reagent in Option C, where it reacts with ethanol to form ethyl chloride.
Phosphorus pentachloride is used as a reagent in Option C.
Topper's Solved these Questions
- HALOALKANES & HALOARENES BOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES & HALOARENES EXERCISE - EFFICIENT
100 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VMC MODULES ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - JEE Main (Archive)
39 Videos
- HALOALKANES AND HALOARENESBOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES AND HALOARENESEXERCISE - IN-CHAPTER EXERCISE - F
6 Videos
Similar Questions
Phosphorus pentachloride in the solid exists as :
View SolutionDissociation of phosphorus pentachloride is favoured by -
View SolutionThe reagents for the following conversion is /are:
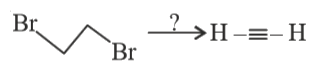
View SolutionThe reagent(s) for the following conversion
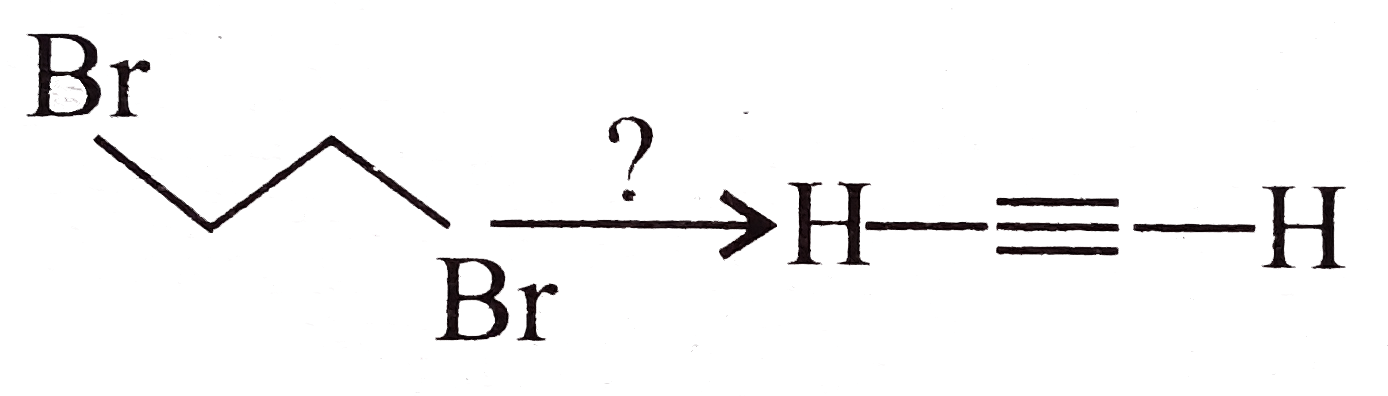
is/are:
View SolutionThe reagent(s) for the following conversion
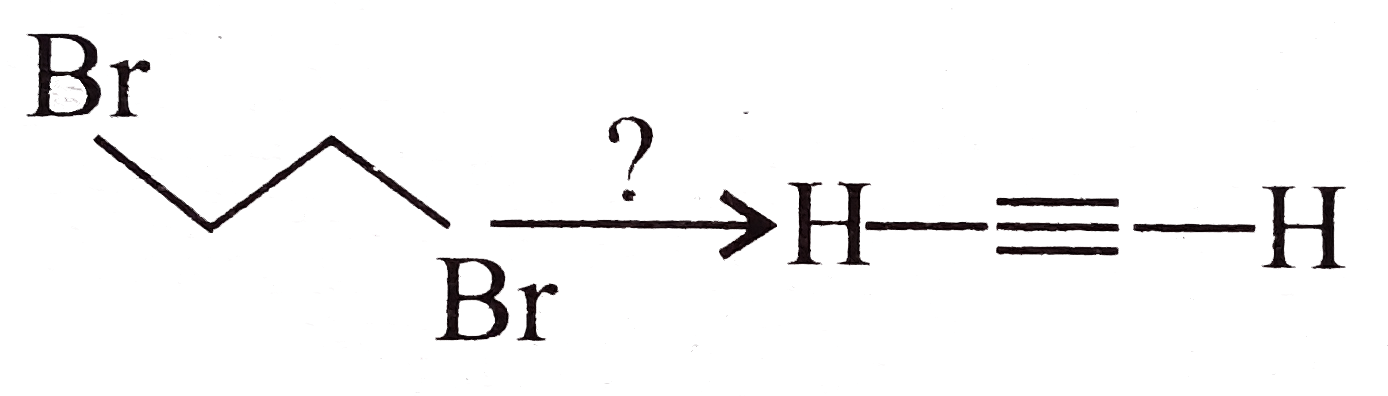
is/are:
View SolutionHow dould you account for the following :
Solid phosphorus pentachloride behaves as an ionic compound.
View SolutionHow do phosphorus pentachloride react with diethyl ether?
View SolutionSuggest a reagent for the following conversion.
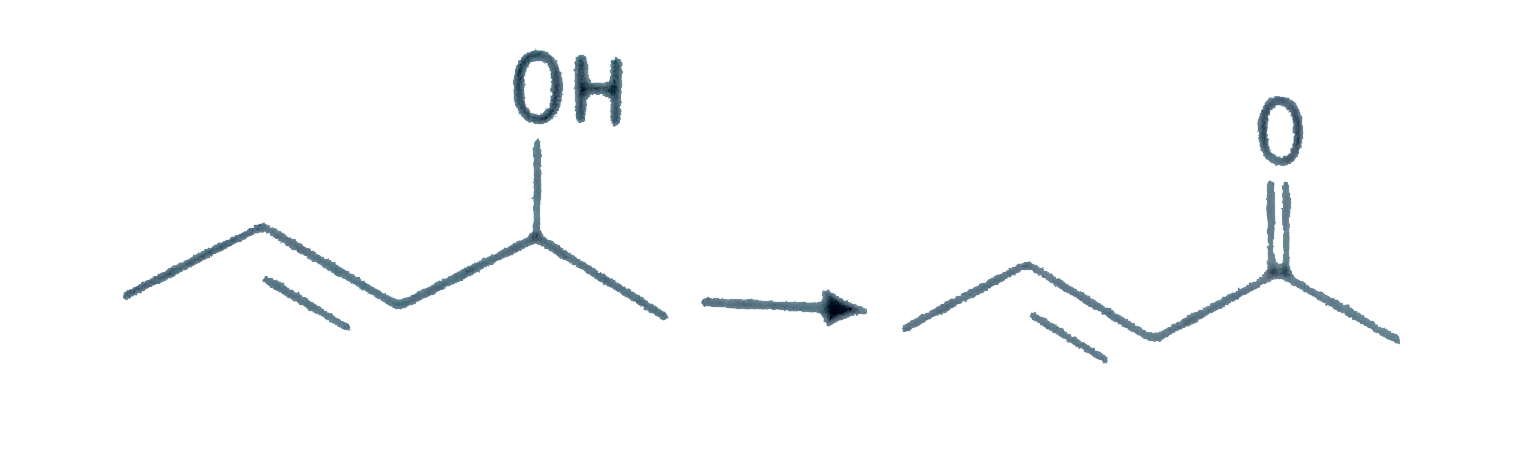
View SolutionThe reagents(s) for the following conversion,
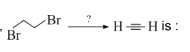 is :
is :
View Solution
Suitable reagent for following conversion
View Solution

