Which of the following compounds does not have an ester linkage ?
Ethyl acid sulphate
Diethyl hydrogen phosphate
Ethyl acetate
CH3CO−O−CO−CH3
The correct Answer is:D
To determine which of the given compounds does not have an ester linkage, we need to understand what an ester linkage is. An ester linkage is formed when a carbon atom is doubly bonded to an oxygen atom (carbonyl group) and also bonded to another oxygen atom that is part of an alkyl or aryl group.
Let's analyze the compounds one by one:
1. Ethyl Acid Sulfate: The structure can be represented as CH3CH2OSO2OH. In this compound, we can identify the ester linkage between the sulfur atom (which is bonded to two oxygen atoms) and the ethyl group (C2H5). Therefore, this compound does have an ester linkage.
2. Diethyl Hydrogen Phosphate: The structure is represented as (C2H5O)2PO(OH). Here, the phosphate group has an oxygen atom bonded to two ethyl groups and one hydroxyl group. The bond between the oxygen and the ethyl group indicates an ester linkage. Thus, this compound also has an ester linkage.
3. Ethyl Acetate: The structure is CH3COOCH2CH3. In this case, we have a carbonyl group (C=O) bonded to an oxygen atom that is also bonded to an ethyl group. This clearly shows an ester linkage. Hence, ethyl acetate does have an ester linkage.
4. Acetic Anhydride: The structure is represented as (CH3CO)2O. In this compound, we have two carbonyl groups (C=O) connected by an oxygen atom, but there is no alkyl or aryl group connected to an oxygen atom in the way that defines an ester linkage. Therefore, acetic anhydride does not have an ester linkage.
From the analysis above, we conclude that the compound that does not have an ester linkage is Acetic Anhydride.
Summary:
The compound that does not have an ester linkage is Acetic Anhydride.
The compound that does not have an ester linkage is Acetic Anhydride.
টপার এই প্রশ্নগুলো সমাধান করেছে
- ALCOHOLS, PHENOLS & ETHERSBOOK - VMC MODULES ENGLISHCHAPTER - ALCOHOLS, PHENOLS & ETHERSEXERCISE - Impeccable
100 Videos
- ALCOHOLS, PHENOLS & ETHERSBOOK - VMC MODULES ENGLISHCHAPTER - ALCOHOLS, PHENOLS & ETHERSEXERCISE - Enable
100 Videos
- ALCOHOLS, PHENOLS & ETHERS BOOK - VMC MODULES ENGLISHCHAPTER - ALCOHOLS, PHENOLS & ETHERS EXERCISE - IN-CHAPTER EXERCISE - L
10 Videos
- ALDEHYDES,KETONES & CARBOXYLIC ACIDS BOOK - VMC MODULES ENGLISHCHAPTER - ALDEHYDES,KETONES & CARBOXYLIC ACIDS EXERCISE - IN-CHAPTER EXERCISE-M
10 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Which of the following compounds does not have any geometrical isomer?
A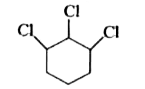 B
B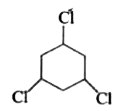 C
C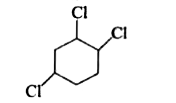 D
D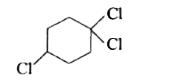
Which of the following compounds does not have any geometrical isomer?
View SolutionWhich of the following compound does not give Tollen's test ?
View SolutionWhich of the following compound does not have hydrogen bonding :
View SolutionWhich of the following compound does not have allylic chloride
View SolutionWhich of the following does not have many segments ?
View SolutionWhich of the following ions does not have S-S linkage?
View SolutionWhich of the following ions does not have S-S linkage?
View Solution

