Hydrolysis of trichloro methane with aqueous KOH gives
Methanol
Ethanol
Acetic acid
Formic acid
The correct Answer is:D
To solve the question regarding the hydrolysis of trichloromethane (CHCl3) with aqueous KOH, we can follow these steps:
1. Identify the Reactants:
- The reactant is trichloromethane (CHCl3), also known as chloroform.
- The other reactant is aqueous KOH, which provides hydroxide ions (OH⁻) for the reaction.
2. Understand the Reaction Type:
- The reaction is a nucleophilic substitution reaction. In this case, the hydroxide ions from KOH will act as nucleophiles and substitute the chlorine atoms in trichloromethane.
3. Substitution of Chlorine Atoms:
- Trichloromethane has three chlorine atoms attached to a carbon atom. During hydrolysis, each chlorine atom will be replaced by a hydroxyl group (OH).
- The reaction can be represented as:
CHCl3+KOH→C(OH)3+KCl
- However, this intermediate (C(OH)₃) is unstable.
4. Formation of Formic Acid:
- The intermediate compound (C(OH)₃) will lose a water molecule (H2O) to achieve stability.
- This leads to the formation of a carbonyl group (C=O) and a hydroxyl group (OH), resulting in the formation of formic acid (HCOOH).
- The overall reaction can be summarized as:
CHCl3+KOH→HCOOH+KCl+H2O
5. Final Product:
- The final product of the hydrolysis of trichloromethane with aqueous KOH is formic acid (HCOOH).
Conclusion:
The hydrolysis of trichloromethane with aqueous KOH gives formic acid (HCOOH).
- The reactant is trichloromethane (CHCl3), also known as chloroform.
- The other reactant is aqueous KOH, which provides hydroxide ions (OH⁻) for the reaction.
- The reaction is a nucleophilic substitution reaction. In this case, the hydroxide ions from KOH will act as nucleophiles and substitute the chlorine atoms in trichloromethane.
- Trichloromethane has three chlorine atoms attached to a carbon atom. During hydrolysis, each chlorine atom will be replaced by a hydroxyl group (OH).
- The reaction can be represented as:
CHCl3+KOH→C(OH)3+KCl
- However, this intermediate (C(OH)₃) is unstable.
- The intermediate compound (C(OH)₃) will lose a water molecule (H2O) to achieve stability.
- This leads to the formation of a carbonyl group (C=O) and a hydroxyl group (OH), resulting in the formation of formic acid (HCOOH).
- The overall reaction can be summarized as:
CHCl3+KOH→HCOOH+KCl+H2O
- The final product of the hydrolysis of trichloromethane with aqueous KOH is formic acid (HCOOH).
The hydrolysis of trichloromethane with aqueous KOH gives formic acid (HCOOH).
Topper's Solved these Questions
- HALOALKANES & HALOARENES BOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES & HALOARENES EXERCISE - IMPECCABLE
100 Videos
- HALOALKANES & HALOARENES BOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES & HALOARENES EXERCISE - ENABLE
99 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VMC MODULES ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - JEE Main (Archive)
39 Videos
- HALOALKANES AND HALOARENESBOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES AND HALOARENESEXERCISE - IN-CHAPTER EXERCISE - F
6 Videos
Similar Questions
In the reaction C2H5MgBr +
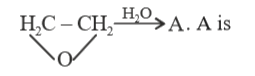
View SolutionWhich of the following is least reactive towards nucleo- philic substitution?
View SolutionWhen rain is accompanied by a thunderstorm, the collected rain water will have a pH value
View SolutionFreon (dichlorodifluoro methane) is used .
View SolutionWhich of the following alkyl halides undergoes hydrolysis with aqueous KOH at the fastest rate ?
View SolutionTrihalogen methane on hydrolysis with NaOH (aq). Gives :
View SolutionHydrolysis of trichloromethane with aqueous KOH gives
View SolutionHydrolysis of trichloromethane with aqueous KOH gives
View Solutionप्राथमिक ऐरोमैटिक ऐमीन को ट्राइक्लोरो मीथेन और ऐल्कोहॉलीय KOH के साथ गर्म करने पर कौन-सा यौगिक प्राप्त होता है ।
View SolutionAlkaline hydrolysis of CH3Br with aqueous KOH is which order reaction /
View SolutionHydrolysis of 1,1, 1-trichloro derivative (A) of alkane gives a molecule (B) on alkaline hydrolysis which produces red coloration with aqueous FeCl3. The compound (A) is -
View SolutionHydrolysis of trichloro methane with aqueous KOH gives
View SolutionHydrolysis of chloroform with aqueous KOH gives finally
View SolutionHydrolysis of tricholoromethane with aqueous KOH gives
View SolutionHydrolysis of chloroform with aqueous KOH gives finally
View SolutionHydrolysis of tricholoromethane with aqueous KOH gives
View Solution

