2.2016 gm of acetaldehyde produceed 13.95 Kcal of heat on combustion. The heat of combustion of CH3CHO will be
279 kcal
972 kcal
27.9 kcal
2.79 kcal
The correct Answer is:A
To find the heat of combustion of acetaldehyde (CH₃CHO), we can follow these steps:
Step 1: Determine the molar mass of acetaldehyde (CH₃CHO)
The molar mass can be calculated as follows:
- Carbon (C): 12 g/mol (2 atoms)
- Hydrogen (H): 1 g/mol (4 atoms)
- Oxygen (O): 16 g/mol (1 atom)
Calculating the molar mass:
Molar mass of CH3CHO=(2×12)+(4×1)+(1×16)=24+4+16=44 g/mol
Step 2: Calculate the number of moles of acetaldehyde in 2.2016 g
Using the formula:
Number of moles=massmolar mass
Substituting the values:
Number of moles=2.2016 g44 g/mol≈0.05005 moles
Step 3: Calculate the heat released per mole of acetaldehyde
We know that 2.2016 g of acetaldehyde releases 13.95 kcal of heat. To find the heat released per mole:
Heat released per mole=Total heat releasedNumber of moles
Substituting the values:
Heat released per mole=13.95 kcal0.05005 moles≈278.5 kcal/mol
Step 4: Round the result
We can round the result to a suitable number of significant figures:
Heat of combustion of CH3CHO≈279 kcal/mol
Final Answer
The heat of combustion of acetaldehyde (CH₃CHO) is approximately 279 kcal/mol.
---
The molar mass can be calculated as follows:
- Carbon (C): 12 g/mol (2 atoms)
- Hydrogen (H): 1 g/mol (4 atoms)
- Oxygen (O): 16 g/mol (1 atom)
Molar mass of CH3CHO=(2×12)+(4×1)+(1×16)=24+4+16=44 g/mol
Using the formula:
Number of moles=massmolar mass
Substituting the values:
Number of moles=2.2016 g44 g/mol≈0.05005 moles
We know that 2.2016 g of acetaldehyde releases 13.95 kcal of heat. To find the heat released per mole:
Heat released per mole=Total heat releasedNumber of moles
Substituting the values:
Heat released per mole=13.95 kcal0.05005 moles≈278.5 kcal/mol
We can round the result to a suitable number of significant figures:
Heat of combustion of CH3CHO≈279 kcal/mol
The heat of combustion of acetaldehyde (CH₃CHO) is approximately 279 kcal/mol.
Topper's Solved these Questions
- CHEMICAL THERMODYNAMICS Book:VMC MODULES ENGLISHChapter:CHEMICAL THERMODYNAMICS Exercise:IN - CHAPTER EXERCISE - J
Explore 10 Videos
- CHEMICAL THERMODYNAMICS Book:VMC MODULES ENGLISHChapter:CHEMICAL THERMODYNAMICS Exercise:IN - CHAPTER EXERCISE - K
Explore 10 Videos
- CHEMICAL THERMODYNAMICS Book:VMC MODULES ENGLISHChapter:CHEMICAL THERMODYNAMICS Exercise:IN - CHAPTER EXERCISE - H
Explore 10 Videos
- CHEMICAL KINETICS Book:VMC MODULES ENGLISHChapter:CHEMICAL KINETICS Exercise:JEE Advanced (Archive)
Explore 51 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:VMC MODULES ENGLISHChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:JEE ADVANCE (ARCHIVE)
Explore 30 Videos
Similar Questions
Knowledge Check
Which one of the following has lowest heat of combustion?
A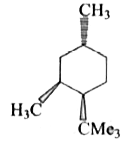 B
B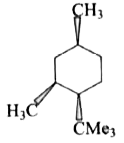 C
C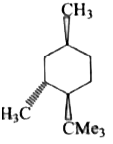 D
D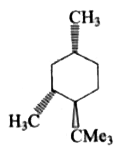
Which of the following has the lowest heat of combustion?
A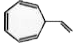 B
B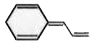 C
C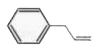 D
D
Find out correct order for heat of combustion of these compounds :
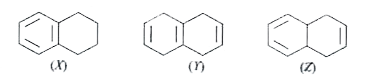 AX>Y>ZBZ>Y>XCY>Z>XDZ>X>Y
AX>Y>ZBZ>Y>XCY>Z>XDZ>X>Y
In the combustion of 0.4 g. of CH4 0.25 Kcal. Of heat is liberated. The heat of combustion of CH4 is
View SolutionIf, combustion of 4g of CH4 liberates 2.5kcal of heat, the heat of combustion of CH4 is :
View SolutionWhich of the following is correct order for heat of combustion?

View SolutionWhich one of the following has the smallest heat of combustion ?
View SolutionWhich is incorrect statement about heats of combustion ?
View Solution
