One mole of N2 and 3 moles of PCl5 are placed in a 100L vessel heated to 227∘C. The equilibrium pressure is 2.05 atm. Assuming ideal behaviour, calculate the degree of dissociation for PCl5andKp for the reaction
PCl5(g)→PCl3(g)+Cl2(g)
Dissociation of PCl5 is written as :
PCl5(g)⇔PCl3(g)+Cl2(g)
Let x be the no. of moles of PCl5 decomposed at equilibrium.
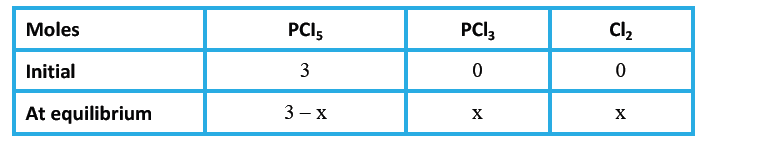
Now total gaseous moles in the container =nT
⇒nT=moles of(PCl5+PCl3+Cl2)+moles ofN2
⇒nT=3−x+x+x+1=4+x
The mixture behaves ideally, hence PV=nTRT
Let us calculate no. of moles by using gas equation
⇒nT=PVRT=2.05×1000.0821×500⇒nT=5
Now, equating the two values of nT, we have:
4+x=5⇒x=1.
⇒ Degree of dissociation =1/3=0.333
Now KP=PPCl3×PCl2PPCl5
PPCl5=3−x4+xp P= equilibrium pressure
=25×2.05=0.82atm
PCl2=PPCl3=x4+xP=15×2.05=0.41 atm
⇒Kp=0.4×0.40.8=0.20 atm
Topper's Solved these Questions
- CHEMICAL EQUILIBRIUMBOOK - VMC MODULES ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - SOLVED EXAMPLES
19 Videos
- CHEMICAL EQUILIBRIUMBOOK - VMC MODULES ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - PRACTICE EXERCISE-1
4 Videos
- CHEMICAL EQUILIBRIUMBOOK - VMC MODULES ENGLISHCHAPTER - CHEMICAL EQUILIBRIUMEXERCISE - IMPECCABLE
50 Videos
- CHEMICAL BONDING-I & IIBOOK - VMC MODULES ENGLISHCHAPTER - CHEMICAL BONDING-I & IIEXERCISE - JEE Advanced (Archive)
98 Videos
- CHEMICAL KINETICS BOOK - VMC MODULES ENGLISHCHAPTER - CHEMICAL KINETICS EXERCISE - JEE Advanced (Archive)
51 Videos
Similar Questions
Consider the reaction
SO2Cl2⇔SO2(g)+Cl2(g)
at 375∘C, the value of equilibrium constant for the reaction is 0.0032. It was observed that the concentration of the three species is 0.050molL−1 each at a certain instant. Discuss what will happen in the reaction vessel?
View SolutionFor 2SO2(g)+O2(g)⇔2SO3(g),Kp=3.4. At an instant, the partial pressures of SO2,O2andSO3 were found to be 0.41 atm, 0.16 atm and 0.57 atm respectively. Comment on the status of equilibrium process.
View SolutionThe equilibrium constant Kp for the thermal dissociation of PCl5 at 200∘C is 1.6 atm. The pressure (in atm) at which it is 50% dissociated at that temperature is
View SolutionState four major physical properties than can be used to distingusish between covalent and ionic compounds Mention the distinguishing feactures in each case .
View SolutionThe rate constant of a reaction depends upon
View SolutionIn the dissociation of PCl_(5) as PCl_(5)(g) hArr PCl_(3)(g)+Cl_(2)(g) If the degree of dissociation is alpha at equilibrium pressure P, then the equilibrium constant for the reaction is
View Solution1 mol of Cl_(2) and 3 mol of PCl_(5) are placed in a 100 L vessel heated to 227^(@)C . The equilibrium pressure is 2.05 atm. Assuming ideal behaviour, calculate the degree of dissociation for PCl_(5) and K_(p) for the reaction. PCl_(5)(g) hArr PCl_(3)(g)+Cl_(2)(g)
View Solutionn mole of PCl_(3) and n mole of Cl_(2) are allowed to react at constant temperature T to have a total equilibrium pressure P , as : PCl_(3)(g)+Cl_(2)(g) hArr PCl_(5)(g) If y mole of PCl_(5) are formed at equilibrium , find K_(p) for the given reaction .
View Solution1 mole of PCl5 taken at 5 atm, dissociates into PCl3 and Cl2 to the extent of 50%
PCl5(g)⇔PCl3(g)+Cl2(g) Thus Kp is :
View SolutionA quantity of PCl_(5) ws heated in a 10 litre vessel at 250^(@)C: PCl_(5_(g))hArrPCl_(3(g))+Cl_(3(g)). At equilibrium the vessel contains 0.1 moles of PCl_(5)0.20 mole of PCl_(3)and 0.2 mole of Cl_(2). The equilibrium constant of the reaction is
View Solutiona' moles of PCl5 are heated in a closed container to equilibrate PCl5(g)⇔PCl3(g)+Cl2(g) at a pressure of p atm . If x moles of PCl5 dissociate at equilibrium , then
View Solutiona' moles of PCl5 are heated in a closed container to equilibriate PCl5(g)⇔PCl3(g)+Cl2(g) at pressure of p atm . If x moles of PCl5 dissociate at equilibrium , then
View SolutionFor a reaction at equilibrium PCl5(g)⇔pcL3(G)+Cl2(g), the degree of dissociation of PCl5 at 2 atm is 0.02. Then the degree of dissociation at 4 atm is
View SolutionA quantity of PCl_(5) was heated in a 10 dm^(3) vessel at 250^(@)C PCl_(5(g)) hArr PCl_(3 (g)) + Cl_(2(g)) . At equilibrium the vessel contains 0.1 mole of PCl_(5) , 0.2 moles of PCl_(3) and 0.2 moles of Cl_2 . The equilibrium constant of the reaction is
View SolutionOne mole of N2 and 3 mole of PCI5 are placed in a 100 litre vessel heated to 227∘c. The equilibrium pressure is 2.05 atm. Assuming ideal behaviour, If Kp of the reaction PCl5⇔PCl3+Cl2 is y×10−1 then what is 'y'?
View SolutionInitially 0.8 mole of PCl_(5) and 0.2 mole of PCl_(3) are mixed in one litre vessel . At equilibrium 0.4 mole of PCl_(3) is present . The value of K_(C) for the reaction PCl_(5(g)) hArr PCl_(3(g)) + Cl_(2(g)) is
View Solution

