A 0.25M solution of pyridinium chloride (C5H5⊕NHClΘ) has pH of 2.89. Calculate pKb for pyridine (C5H5N).
Step by step video solution for A 0.25M solution of pyridinium chloride (C_(5)H_(5)overset(o+)NHCl^(Theta)) has pH of 2.89. Calculate pK_(b) for pyridine (C_(5)H_(5)N). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- IONIC EQUILIBRIUMBOOK - VMC MODULES ENGLISHCHAPTER - IONIC EQUILIBRIUMEXERCISE - PRACTICE EXERCISE - 5
5 Videos
- IONIC EQUILIBRIUMBOOK - VMC MODULES ENGLISHCHAPTER - IONIC EQUILIBRIUMEXERCISE - PRACTICE EXERCISE - 6
6 Videos
- IONIC EQUILIBRIUMBOOK - VMC MODULES ENGLISHCHAPTER - IONIC EQUILIBRIUMEXERCISE - PRACTICE EXERCISE - 3
3 Videos
- INTRODUCTION TO ORGANIC CHEMISTRY BOOK - VMC MODULES ENGLISHCHAPTER - INTRODUCTION TO ORGANIC CHEMISTRY EXERCISE - JEE ADVANCED ARCHIVE
81 Videos
- JEE MAIN - 5BOOK - VMC MODULES ENGLISHCHAPTER - JEE MAIN - 5EXERCISE - PART II : CHEMISTRY (SECTION - 2)
3 Videos
Similar Questions
The solubility product of BaSO4 is 1.5×10−9. Find the solubility of BaSO4 in
pure water
View SolutionCalculate the pH at which an acid indicator with Ka=1.0×10−5 changes colour when the indicator is 1.00×10−3M.
View SolutionA weak acid HA has Ka=10−6. What would be the molar ratio of this acid and its salt with strong base so that pH of the buffer solution is 5?
View SolutionAn isomer of ethanol is:
View SolutionThe theoretical volume of O2(g) that can be obtained by decomposition of 1L of a H2O2 solution at N.T.P is known as its volume strength. Calculate volume strength of 2MH2O2 solution.
View SolutionA 0.25M solution of pyridinium chloride (C5H5⊕NHC1Θ) has pH of 2.89. Calculate pKb for pyridine (C5H5N).
View SolutionSelect the correct answer:
a.
i. PhOC2H5 ii. Ph−−−O−−−Ph
iii. PhI iv. C2H5OC2H5
b.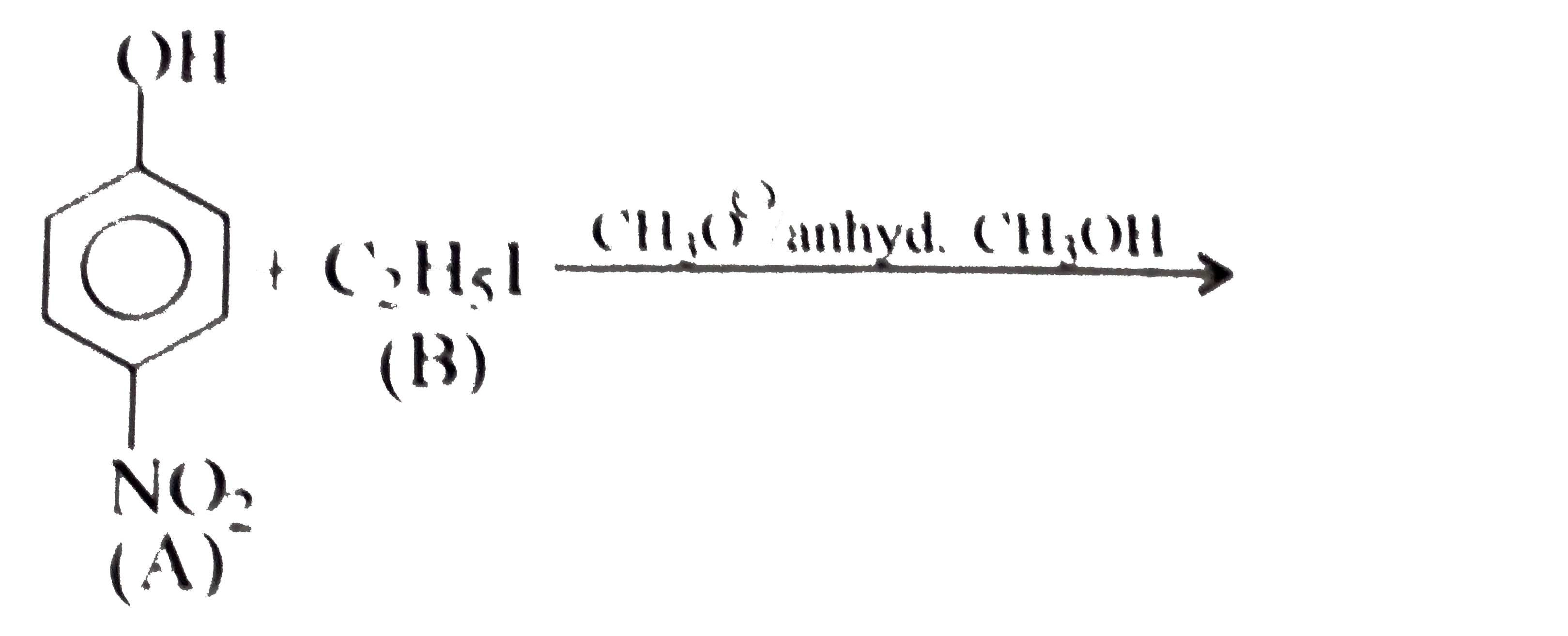
i. p−−NO2−−C6H4−−OC2H5
ii. p−−NO2−−C6H4−−O−−C6H4−−NO2−−p
iii. C2H5−−O−−CH3
iv. p−−NO2−−C6H5−−I.
c.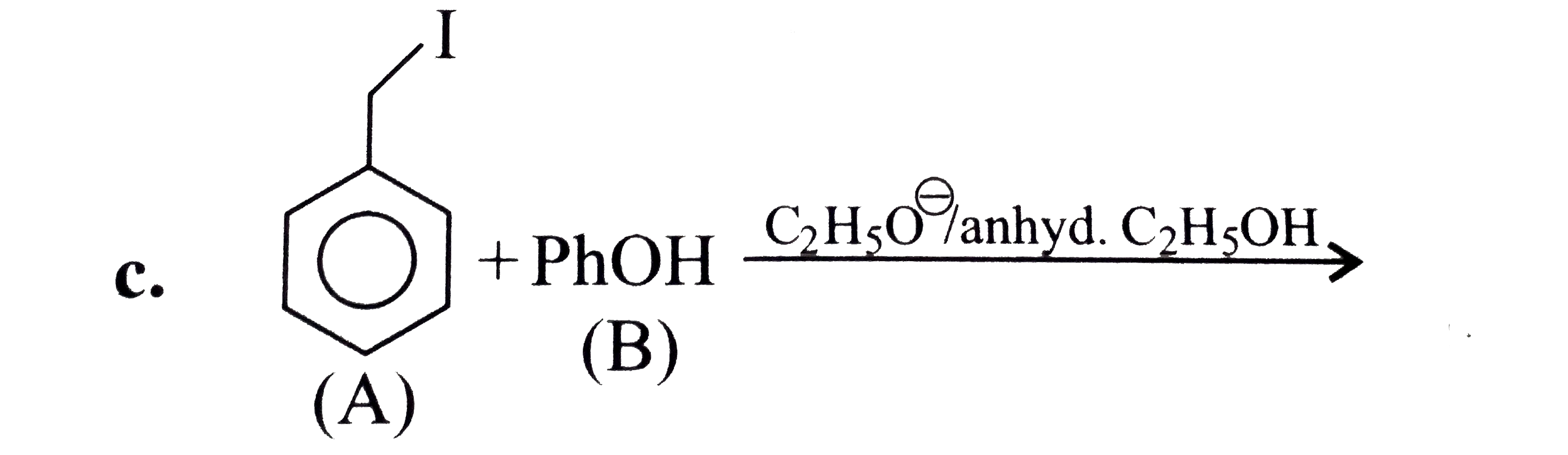
i. C6H5CH2−−O−−Ph
ii. C6H5CH2−−O−−C2H5
iii. Ph−−O−−C2H5
iv. C2H5−−O−−C2H5
d.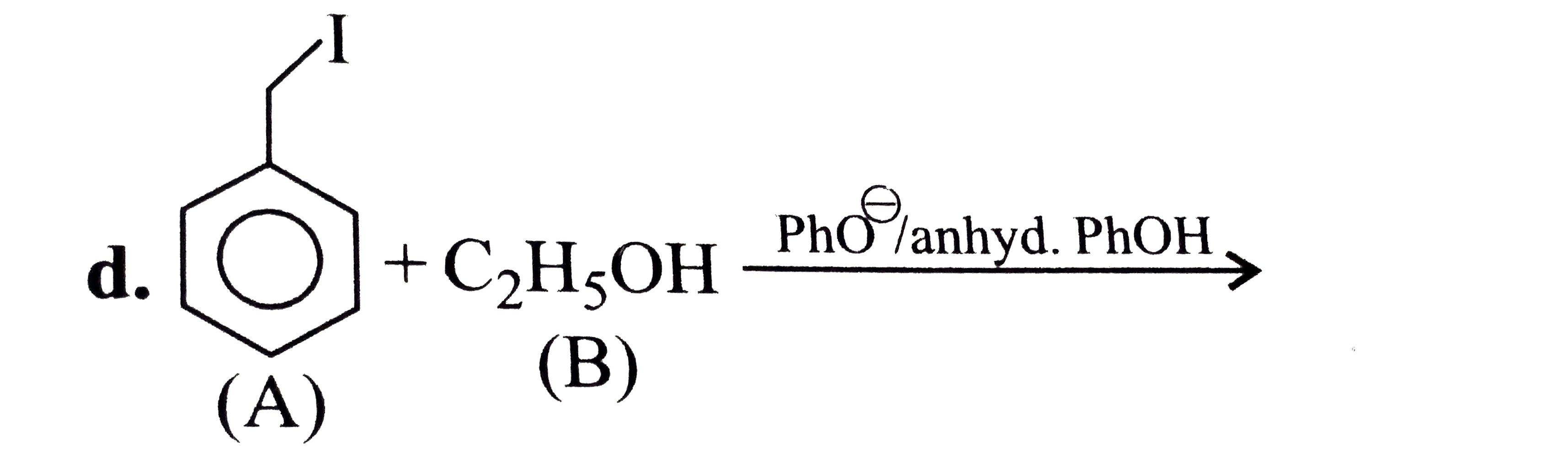
i. C6H5CH2−−O−−C2H5
ii. C6H5−−CH2−−O−−Ph
iii. Ph−−O−−C2H5
iv. C2H5−−O−−C2H5 .
e.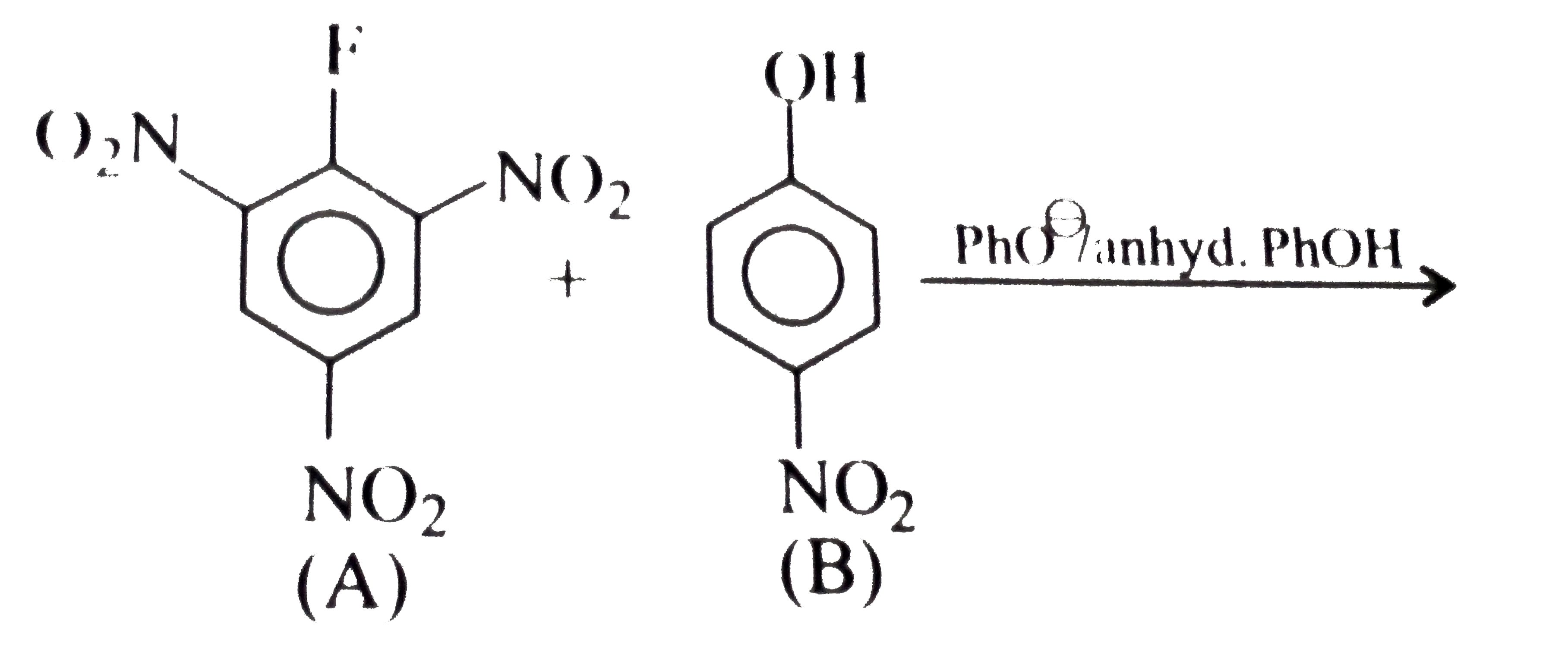
i.
ii.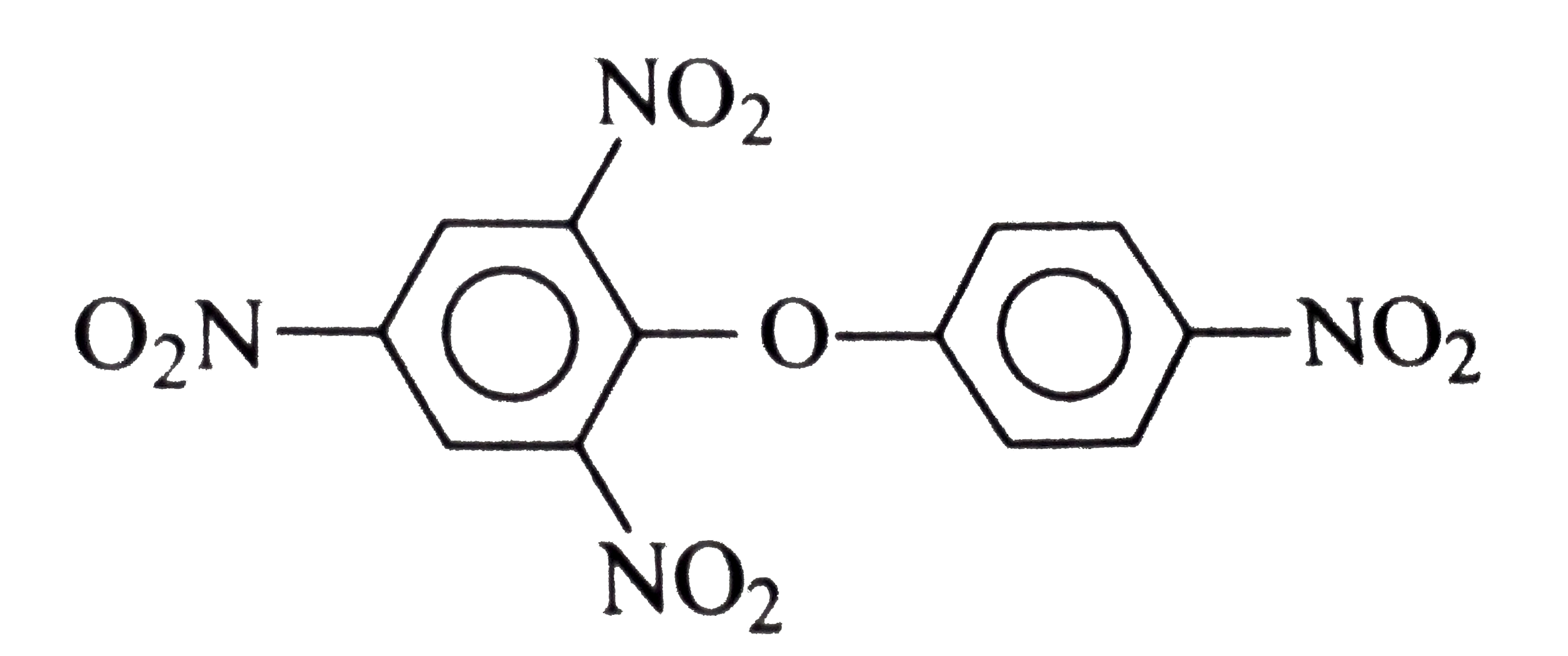
iii.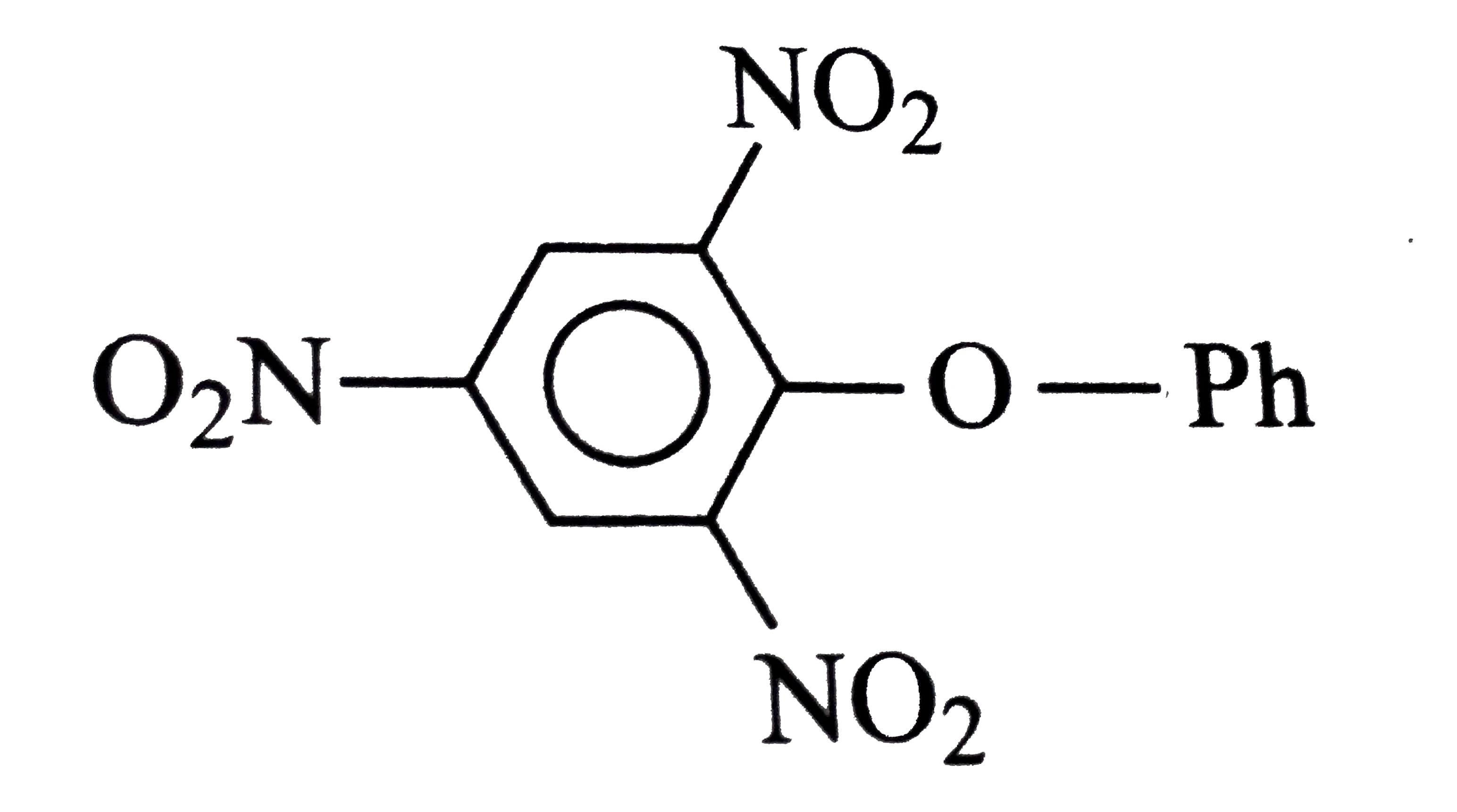
iv.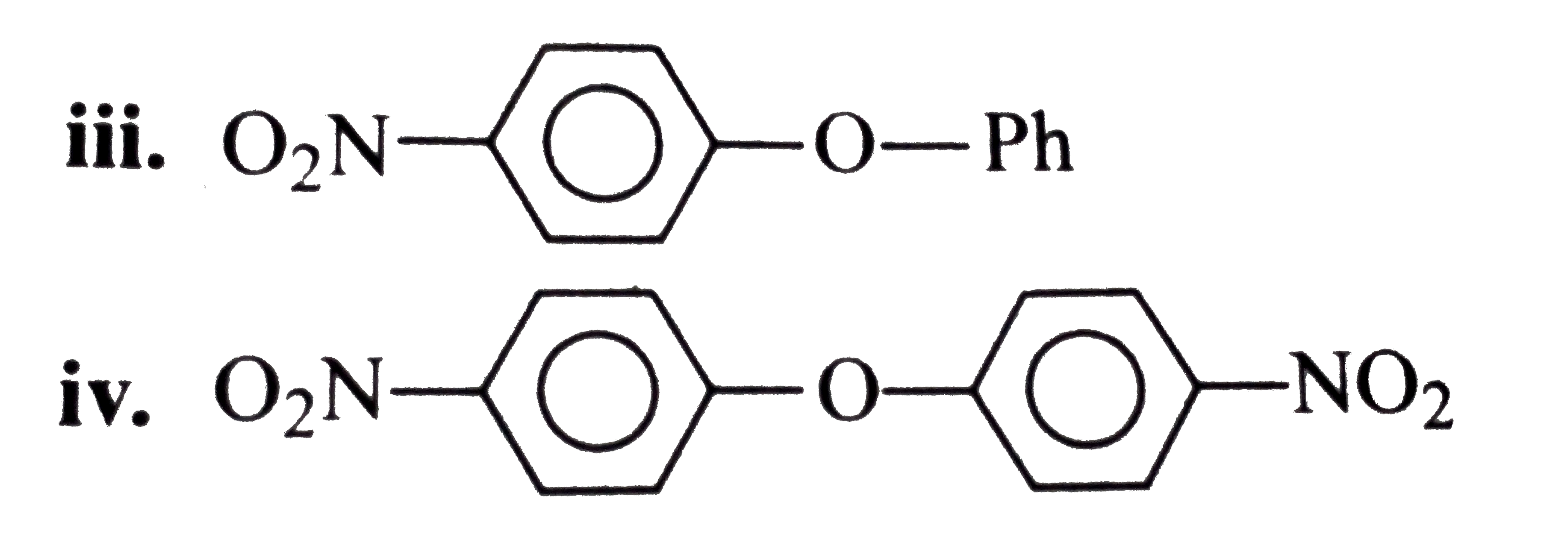
f.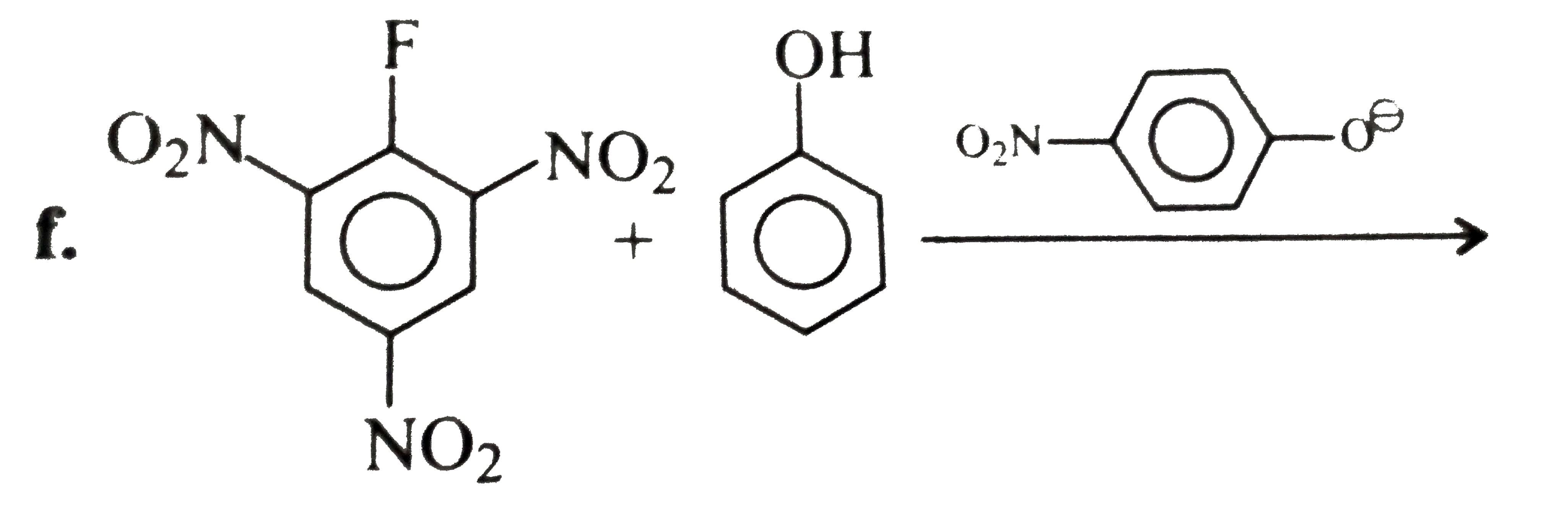
i.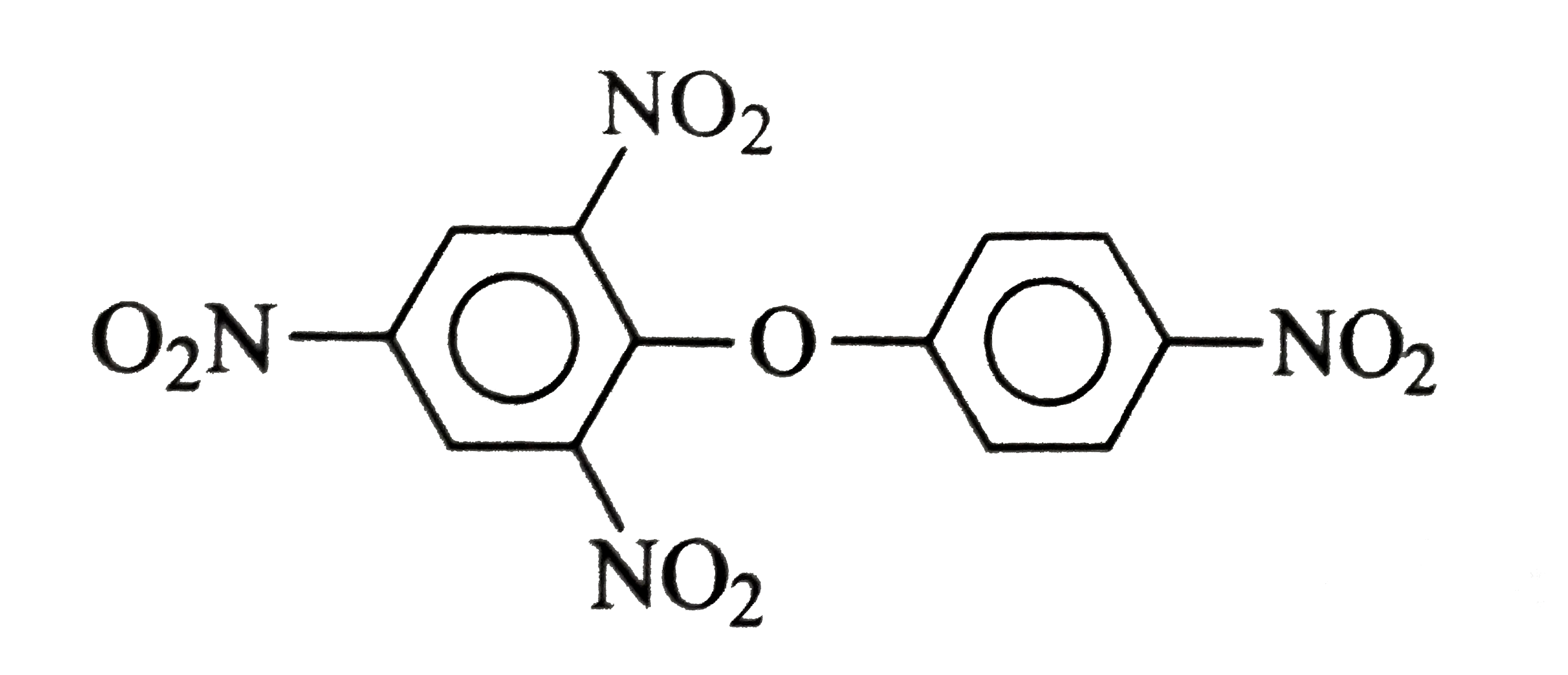
ii.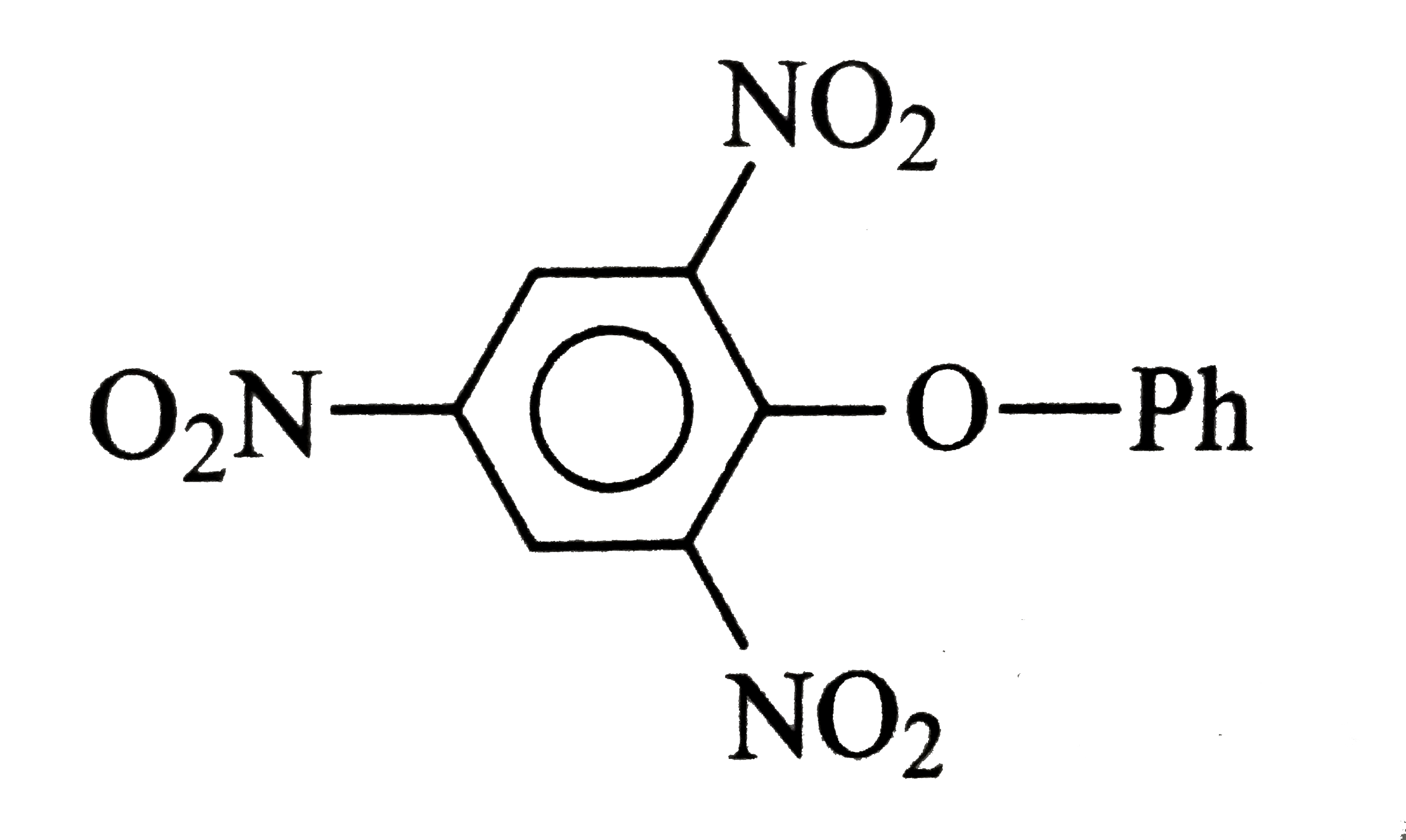
iii.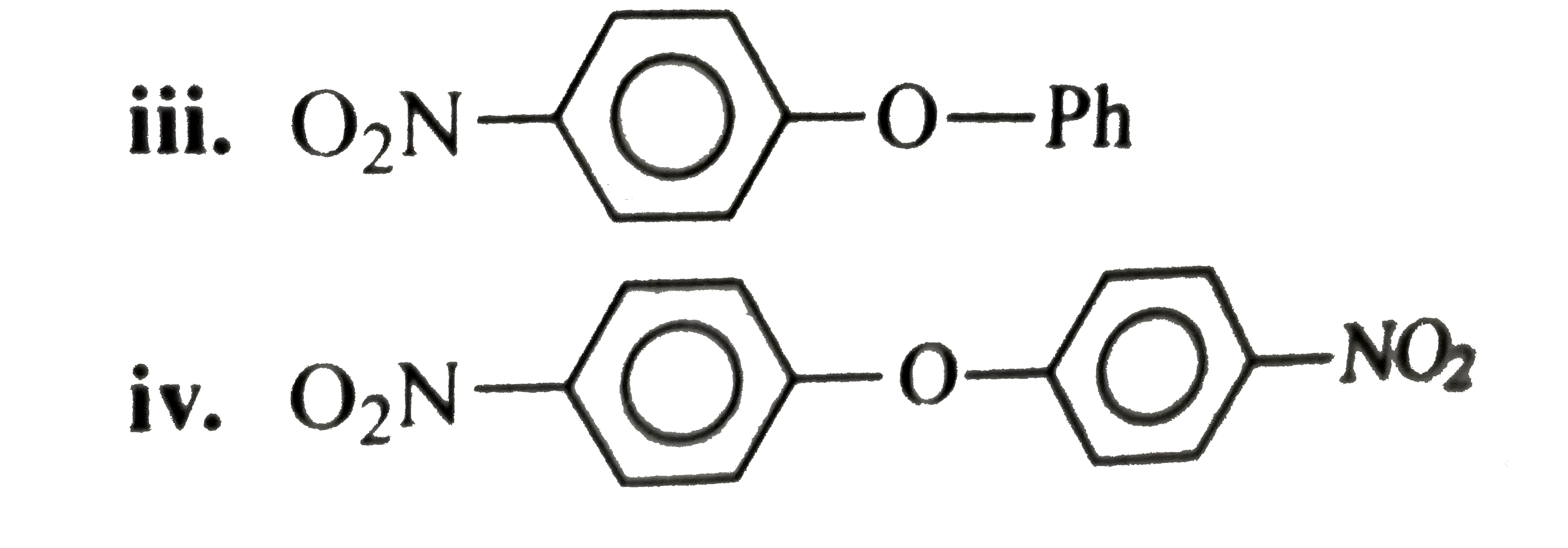
View SolutionEquilibrium constant for the following reaction is 1xx10^(-9) : C_(5)H_(5)N(aq.)+H_(2)O(l)hArrC_(5)H_(5)NH^(+)(aq.)+OH^(-)(aq.) Determine the moles of pyridinium chloride (C_(5)H_(5)N) to obtain a buffer solution of pH=5 :
View SolutionA 0.25M solution of pyridinium chloride C5H5NH+Cl− was found to have a pH of 2.75 What is Kb for pyridine, C5H5N?
View SolutionThe percentage of pyridine (C_(5)H_(5)N) that forms pyridinium ion (C_(5)H_(5)N^(+)H) in a 0.10 M aqueous pyridine solution ( K_(b) for C_(5)H_(5)N=1.7xx10^(-9) ) is
View SolutionThe percentage of pyridine (C_(2)H_(5)N) that forms pyridinium ion (C_(2)H_(5)N^(+)H) in a 0.10 M aqueous pyridine solution ( K_(b) for C_(2)H_(5)N = 1.7 xx 10^(-9) ) is
View SolutionThe percentage of pyridine (C_(5)H_(5)O) that forms pyridinium ion (C_(5)H_(5) overset(+)NH) in a 0.10 M aqueons pyridine solution is (K_(b) for C_(5)H_(5)N=1.7 xx 10^(-9))
View SolutionSelect the IUPAC name of the following
C2H5−N∣C2H5−O∣∣S∣∣O−C6H5
View SolutionThe percentage of pyridine (C_(5) H_(5) N) that forms pyridinium ion (C_(5) H_(5) N^(+) H) in a 0.10 M aqueous pyridine solution ( K_(b) for C_(5) H_(5) N = 1.7 xx 10^(-9) ) is -
View SolutionA0.25 M solution of pyridinium chloride, C5H5NH+Cl−, was found to have a pH of 2.89 . What is the Kb for pyridine, C5H5N ?
View SolutionIdentify A from the following: (i) C_(6)H_(5)COCH_(3) overset(A)to C_(6)H_(5)COOH+CO_(2)+H_(2)O (ii) C_(6)H_(5)COCH_(3) overset(A)to C_(6)H_(5)-underset(OH)underset(|)overset(H)overset(|)C-C_(6)H_(5) (iii) C_(6)H_(5)COCH_(3) overset(A)to C_(6)H_(5)CH_(2)CH_(3)+H_(2)O
View Solution

