The values of dissociation constants of some acids ( at 25∘C are as follows. Indicate which is the strongest acid in water
1.4×10−2
1.6×10−4
4.4×10−10
4.3×10−7
The correct Answer is:1
To determine which acid is the strongest in water based on their dissociation constants (Ka), we can follow these steps:
1. Understand the Concept of Acid Strength: The strength of an acid in water is determined by its ability to dissociate and produce hydrogen ions (H⁺). The more H⁺ ions produced, the stronger the acid.
2. Identify the Dissociation Constants (Ka): We need to look at the given dissociation constants (Ka) for the acids. The larger the value of Ka, the stronger the acid.
3. Compare the Ka Values: The provided Ka values are:
- Acid 1: 10−2
- Acid 2: 10−4
- Acid 3: 10−10
- Acid 4: 10−7
4. Determine the Maximum Ka: Among the given values, 10−2 is the largest. This indicates that Acid 1 has the highest tendency to dissociate and produce H⁺ ions.
5. Conclusion: Since Acid 1 has the highest Ka value, it is the strongest acid in water.
Final Answer:
The strongest acid in water is the one with the dissociation constant 10−2.
---
- Acid 1: 10−2
- Acid 2: 10−4
- Acid 3: 10−10
- Acid 4: 10−7
The strongest acid in water is the one with the dissociation constant 10−2.
Topper's Solved these Questions
- IONIC EQUILIBRIUMBook:VMC MODULES ENGLISHChapter:IONIC EQUILIBRIUMExercise:IN - CHAPTER EXERCISE - D
Explore 10 Videos
- IONIC EQUILIBRIUMBook:VMC MODULES ENGLISHChapter:IONIC EQUILIBRIUMExercise:IN - CHAPTER EXERCISE - E
Explore 10 Videos
- IONIC EQUILIBRIUMBook:VMC MODULES ENGLISHChapter:IONIC EQUILIBRIUMExercise:IN - CHAPTER EXERCISE - B
Explore 10 Videos
- INTRODUCTION TO ORGANIC CHEMISTRY Book:VMC MODULES ENGLISHChapter:INTRODUCTION TO ORGANIC CHEMISTRY Exercise:JEE ADVANCED ARCHIVE
Explore 81 Videos
- JEE MAIN - 5Book:VMC MODULES ENGLISHChapter:JEE MAIN - 5Exercise:PART II : CHEMISTRY (SECTION - 2)
Explore 3 Videos
Similar Questions
Knowledge Check
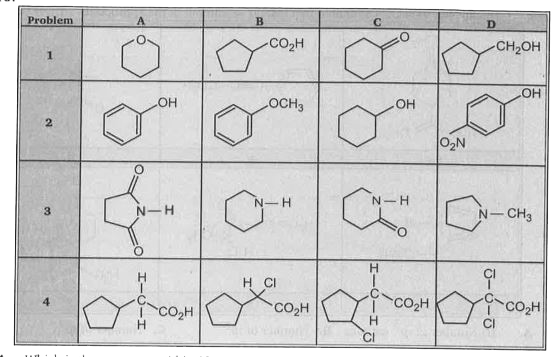
Which is the strongest acid in 1?AABBCCDD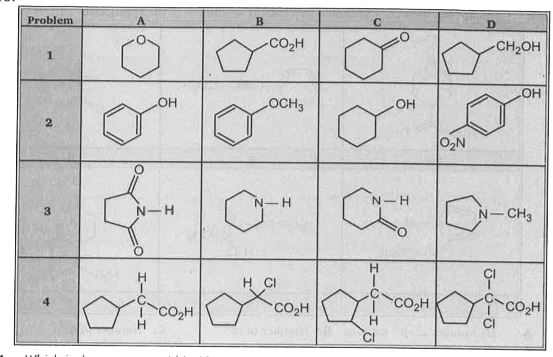
Which is the strongest acid in 2 ?AABBCCDD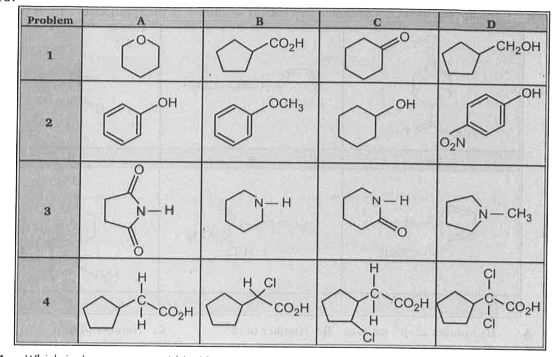
Which is the strongest acid in 4 ?AABBCCDD
