Given that ΔTf is the depression in freezing point of the solvent in a solution of a non-volatile solute of molarity m,the quantity Ltm→0(ΔTf/m) is equal to ……………. .
Zero
One
Three
None of the above
The correct Answer is:D
Step by step video solution for Given that DeltaT_(f) is the depression in freezing point of the solvent in a solution of a non-volatile solute of molarity m,the quantity underset(m rarr0)(Lt) (DeltaT_(f)//m) is equal to ……………. . by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- LIQUID SOLUTIONSBOOK - VMC MODULES ENGLISHCHAPTER - LIQUID SOLUTIONSEXERCISE - IN-CHAPTER EXERCISE -I
10 Videos
- LIQUID SOLUTIONSBOOK - VMC MODULES ENGLISHCHAPTER - LIQUID SOLUTIONSEXERCISE - IN-CHAPTER EXERCISE -J
10 Videos
- LIQUID SOLUTIONSBOOK - VMC MODULES ENGLISHCHAPTER - LIQUID SOLUTIONSEXERCISE - IN-CHAPTER EXERCISE -G
9 Videos
- LIQUID SOLUTION BOOK - VMC MODULES ENGLISHCHAPTER - LIQUID SOLUTION EXERCISE - IMPECCABLE
100 Videos
- MOCK TEST 1BOOK - VMC MODULES ENGLISHCHAPTER - MOCK TEST 1EXERCISE - PART II : CHEMISTRY (SECTION-2)
10 Videos
Similar Questions
The osmotic pressure of 5% (mass/volume) solution of cane sugar at 50-C is
View SolutionWhich of the following is not a colligative property ?
View SolutionThe molal depression constant for water is 1.86∘C. The freezing point of a 0.05−molal solution of a non-electrolyte in water is
View SolutionWhat is the mole fraction of the solute in a 1.00 m aqueous solution ?
View SolutionThe oxidation number of phosphorus in ATP (adenosine triphosphate) is
View SolutionGiven that ΔTf is the depression in freezing point of the solvent in a solution of a non-volatile solute of molarity m,the quantity Ltm→0(ΔTf/m) is equal to ……………. .
View SolutionFreezing Point Depression: when 15.0grams of ethyl alcohol (C2H5OH) is dissolved in 750grams of formic acid, the freezing point of the solution is 7.20∘C. The freezing point of pure formic acid is 8.40∘C Evalulate Kf for formic acid.
Strategy: First calculate the molality and the depression of the freezing point. Then solve the equation ΔTf=Kfm for Kf by substituting values for m and ΔTf.
View SolutionTwo solutions of non-volatile and non-electrolyte solute A and B are prepared separately. The molar mass ratio MAMB=13. Both are prepared as 5% by weight solution in water. Then what is the ratio of freezing point depresions, (ΔTf)A(ΔTf)B of the solutions?
View SolutionFigure explains elevation in boiling point when a non-volatile solute is added to a solvent. Variation of vapour pressure with temperaure and elevation in boiling point is marked.
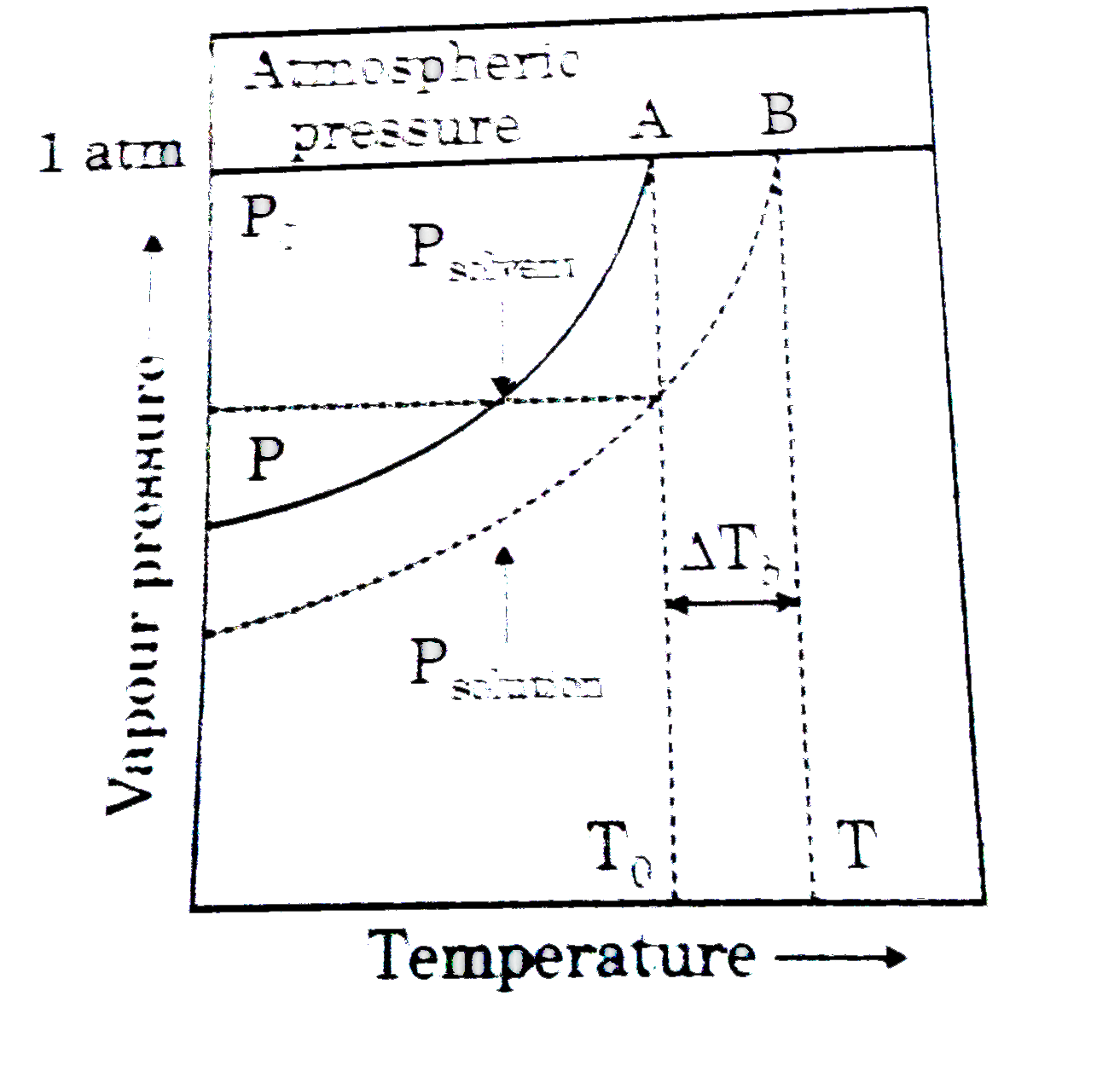
Given that ΔTb is the elevation in boiling point of the solvent in a solution of molarity m then limm→0(ΔTbm) is equal to:
View SolutionIf all symbols have their usual meaning, then for a non-volatile non-electrolyte solute Ltm→0(ΔTfm) is equal to
View SolutionGiven that Delta T_(f) is the depression in freezing point of the solvent in a solution of a non-volatile solute of molality m, the quantity lim_(m rarr 0)((Delta T_(f))/(m)) is equal to
View Solutionयदि ΔTb क्वथनांक में उन्नयन तथा m विलयन की मोललता को दर्शाता हो तो सिद्ध कीजिए कि,
ΔTb=Kb⋅m
View Solutionइकाई मोललता के अवाष्पशील विलेय से बने विलयन में विलायक के हिमांक में अवनमन है, राशि limm→0(ΔTfm) किसके बराबर होगी?
View Solutionयदि ΔTb क्वथनांक में उन्नयन तथा m विलयन की मोललता को दर्शाता हो तो सिद्ध कीजिए कि,
ΔTb=Kb⋅m
View SolutionConsider following figure and answer the questions at the end of it. Figure explains elevation in boiling point when a non-volatile solute is added to a solvent. Variation of vapour pressure with temperature and showing elevation in boiling point. Given that DeltaT_(b) is the elevation in boiling point of the solvent in a solution of molality m then underset(mto0)(Lt)((DeltaT_(b))/(m)) is equal to
View Solutionएक अवाष्पशील विलेय के विलयन (जिसकी मोललता m है) में विलायक के हिमांक में अवनमन Tf है। तब Ltm→0(ΔTfm) का मान. ..... के बराबर होगा।
View Solution

