Alkyl halides react with dialkyl copper reagents to give:
Alkenes
Alkyl copper halides
Alkanes
Alkenyl halides
The correct Answer is:3
To solve the question regarding the reaction of alkyl halides with dialkyl copper reagents, we can break it down into a step-by-step explanation.
Step 1: Understanding the Reactants
- Alkyl Halides: These are organic compounds containing carbon and halogen atoms (from group 17 of the periodic table: fluorine, chlorine, bromine, or iodine). They can be represented as R-X, where R is an alkyl group and X is a halogen.
- Dialkyl Copper Reagents: These are organocopper compounds represented as R2CuLi, where R is an alkyl group. A common example is lithium diethylcuprate (C2H5)2CuLi, also known as Gilman reagent.
Step 2: The Reaction Mechanism
- When an alkyl halide (R'-X) reacts with a dialkyl copper reagent (R2CuLi), a nucleophilic substitution reaction occurs.
- The copper reagent acts as a nucleophile, where one of the alkyl groups (R) from the dialkyl copper reagent attacks the carbon atom of the alkyl halide (R'-X), leading to the formation of a new carbon-carbon bond.
Step 3: Formation of Products
- The reaction results in the formation of a new alkane (R-R') and a by-product of copper halide (R-Cu) and lithium halide (LiX).
- The general reaction can be summarized as:
R2CuLi+R′−X→R−R′+R−Cu+LiX
- Here, R-R' is the newly formed alkane, and R-Cu and LiX are the side products.
Step 4: Example of the Reaction
- For instance, if we take ethyl iodide (C2H5I) as the alkyl halide and lithium diethylcuprate ((C2H5)2CuLi) as the dialkyl copper reagent, the reaction can be illustrated as follows:
(C2H5)2CuLi+C2H5I→C4H10+(C2H5)Cu+LiI
- In this case, butane (C4H10) is formed as the main product.
Conclusion
- The overall conclusion is that when alkyl halides react with dialkyl copper reagents, they yield alkanes as the primary product.
Final Answer
- Alkyl halides react with dialkyl copper reagents to give: Alkanes.
---
- Alkyl Halides: These are organic compounds containing carbon and halogen atoms (from group 17 of the periodic table: fluorine, chlorine, bromine, or iodine). They can be represented as R-X, where R is an alkyl group and X is a halogen.
- Dialkyl Copper Reagents: These are organocopper compounds represented as R2CuLi, where R is an alkyl group. A common example is lithium diethylcuprate (C2H5)2CuLi, also known as Gilman reagent.
- When an alkyl halide (R'-X) reacts with a dialkyl copper reagent (R2CuLi), a nucleophilic substitution reaction occurs.
- The copper reagent acts as a nucleophile, where one of the alkyl groups (R) from the dialkyl copper reagent attacks the carbon atom of the alkyl halide (R'-X), leading to the formation of a new carbon-carbon bond.
- The reaction results in the formation of a new alkane (R-R') and a by-product of copper halide (R-Cu) and lithium halide (LiX).
- The general reaction can be summarized as:
R2CuLi+R′−X→R−R′+R−Cu+LiX
- Here, R-R' is the newly formed alkane, and R-Cu and LiX are the side products.
- For instance, if we take ethyl iodide (C2H5I) as the alkyl halide and lithium diethylcuprate ((C2H5)2CuLi) as the dialkyl copper reagent, the reaction can be illustrated as follows:
(C2H5)2CuLi+C2H5I→C4H10+(C2H5)Cu+LiI
- In this case, butane (C4H10) is formed as the main product.
- The overall conclusion is that when alkyl halides react with dialkyl copper reagents, they yield alkanes as the primary product.
- Alkyl halides react with dialkyl copper reagents to give: Alkanes.
টপার এই প্রশ্নগুলো সমাধান করেছে
- HALOALKANES AND HALOARENESBOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES AND HALOARENESEXERCISE - IN-CHAPTER EXERCISE - F
6 Videos
- HALOALKANES AND HALOARENESBOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES AND HALOARENESEXERCISE - IN - CHAPTER EXERCISE - D
5 Videos
- HALOALKANES & HALOARENES BOOK - VMC MODULES ENGLISHCHAPTER - HALOALKANES & HALOARENES EXERCISE - IMPECCABLE
100 Videos
- HYDROCARBONSBOOK - VMC MODULES ENGLISHCHAPTER - HYDROCARBONSEXERCISE - JEE Advanced Archive
121 Videos
সম্পর্কিত প্রশ্ন
Freon (dichlorodifluoro methane) is used .
View SolutionEthyl bromide can be converted into ethyl alcohol by :
View SolutionIn the reaction C2H5MgBr +
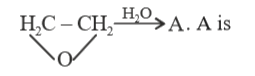
View SolutionGive one characteristic test which would distinguish CH4 from C2H2.
View SolutionAlkyl halides reacts with dialkyl copper reagents to give (A) . The reaction is called B,(A) and (B) are :
View SolutionAlkyl halides react with dialkyl lithium cuprate to give
View SolutionAniline reacts with alkyl halide to give
View SolutionAlkyl halides react with……reagents to give alkanes
View SolutionAniline reacts with excess alkyl halide to give
View SolutionAlkyl halides react with dialkyl copper lithium reagents to give
View Solutionएक ऐल्किल हैलाइड की सिल्वर नाइट्राइट से क्रिया करने पर एक नाइट्रोऐल्किल प्राप्त होता है जबकि एक ऐल्किल हैलाइड की सोडियम नाइट्राइट से क्रिया करने पर एक ऐल्किल नाइट्राइट प्राप्त होता है, क्यों?
View SolutionGrignard reagent is formed when alkyl halide react with which one of the following
View SolutionAniline reacts with excess of alkyl halide to give
View Solutionएल्किल हैलाइडें डाइएल्किल कॉपर अभिकर्मकों के साथ अभिक्रिया करके देते हैं
View SolutionAlkyl halides react with lithium dialkyl copper reagents to give :
View Solutionएल्किल हैलाइड डाइएल्किलकॉपर अभिकर्मकों के साथ अभिक्रिया करके देते हैं
View Solution

