What is the edge length of a cube whose volume is 4,096cm3 ?
Video Solution
Text Solution
The correct Answer is:2
Number of milimoles =10ml×10−3M=10−2
Number of moles =10−5
Now, number of molecules =10−5×6×1023=6×1018
∵ Surface area occupied by 6×1018 molecules =0.24 cm2
∵ Surface area occupied by 1 molecule =0.246×1018 cm2=0.04×10−18cm2
As, it is given that polar head is a cube
So, surface area of cube =a2 (a = edge length)
a2=0.04×10−18cm2
a=2×10−10cm=2 pm
|
Topper's Solved these Questions
- MOCK TEST 8Book:VMC MODULES ENGLISHChapter:MOCK TEST 8Exercise:CHEMISTRY (SECTION 2)
Explore 5 Videos
- MOCK TEST 7Book:VMC MODULES ENGLISHChapter:MOCK TEST 7Exercise:CHEMISTRY (SECTION 2)
Explore 5 Videos
- MOCK TEST 9Book:VMC MODULES ENGLISHChapter:MOCK TEST 9Exercise:CHEMISTRY (SECTION 2)
Explore 5 Videos
Similar Questions
Knowledge Check
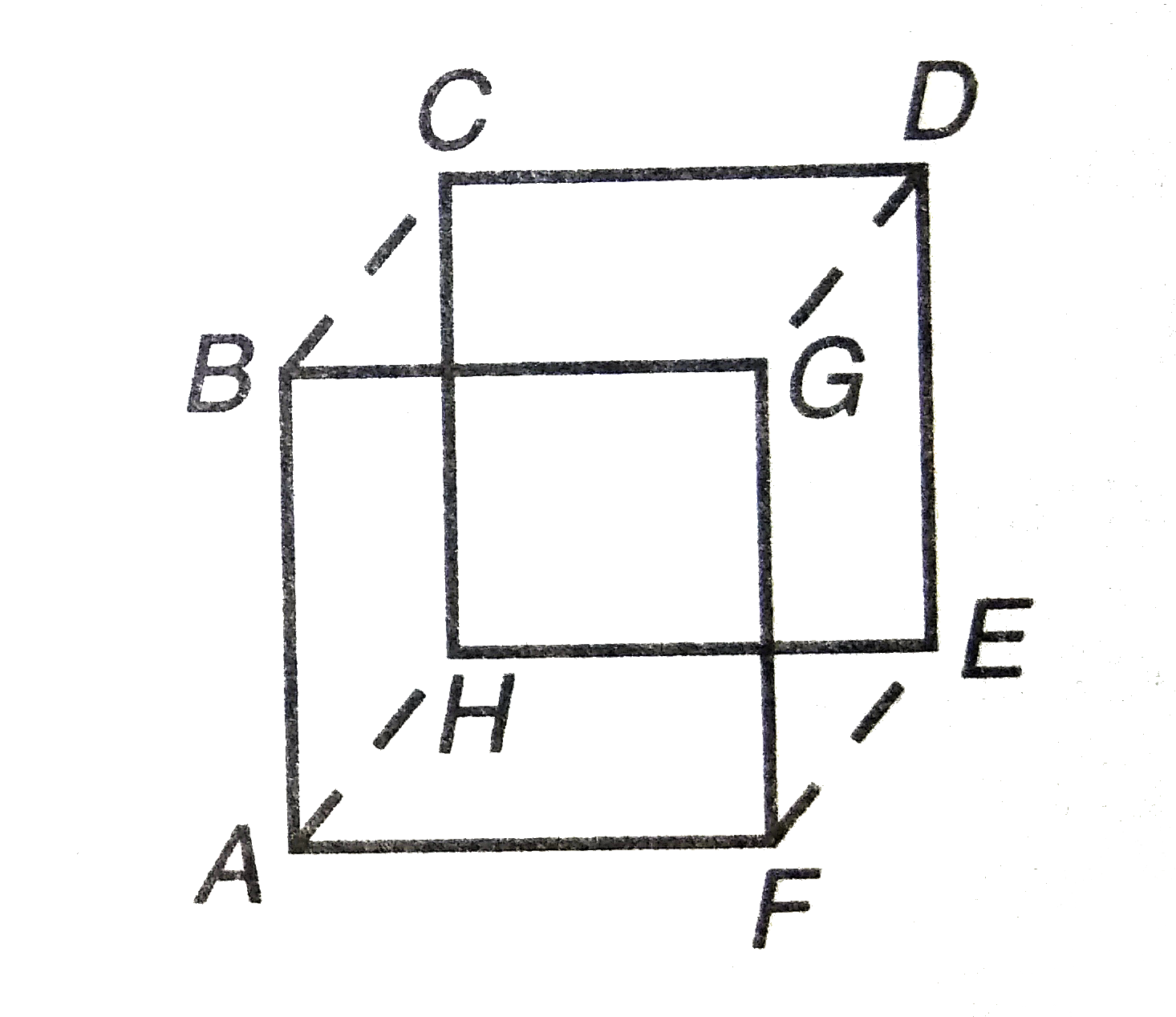
In the figure above, if the edge length of the cube is 4, what is the shortest distance from A to D?A4√2B4√3C8D4√2÷4In cubic meters , what is the volume of a large cube whose edges each measure 6 meters in length ?
A18B36C64D216The unit cell of aluminium is a cube with an edge length of 405 pm. The density of aluminium is 2.70gcm−3 . What type of unti celll of alluminium is ?
ABody centred cubic cellBFace-centred cubic cellCEnd-centred cubic cellDSimple cubic cell
A cube whose volume is 1/8 cubic centimetre is placed on top of a cube whose volume is 1 cm3. The two cubes are then placed on top of a third cube whose volume is 8 cm3. The height of the stacked cubes is (a) 3.5 cm (b) 3 cm (c) 7 cm (d) none of them
View SolutionFind the length of each edge of a cube. If its volume is
216cm3
(ii) 1.728m3
View SolutionFind the surface area of a cube whose edge is 11cm.
View SolutionIf the sum of all the edges of a cube is 36 cm, then the volume (in cm3) of that cube is (a) 9 (b) 27 (c) 219 (d) 729
View SolutionIf the length of an edge of cube A is one-third the length of an edge of cube B, what is the ratio of the volume of cube A to the volume of cube B?
View Solution
