The maximum amount of BaSO4 precipitated on mixing 20 mL of 0.5 M BaCl2 with 20 mL of 1 M H2SO4 is
A
0.25 mole
B
0.5 mole
C
1 mole
D
0.01 mole.
Text Solution
The correct Answer is:D
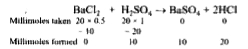
⇒ 10 millimoles ( 0.01 mol ) of BaSO_4 are formed
|
Similar Questions
Knowledge Check
The maximum amount of BaSO4 precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1M) will correspond to
A1.0 MB0.5 MC1.5 MD2.0 MThe maximum amount of BaSO4 precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1M) will correspond to
A0.5 MB1.0 MC18 molesD100 molesThe maximum amount of BaSO4 that can be obtained on mixing 0.5 mole BaCl2 with 1 mole H2SO4 is:
A0.5molB0.1molC0.15molD0.2mol
The maximum amount of BaSO4 precipitated on mixing BaCl2(0.5M) with H2SO4(1M) will correspond to :
View SolutionThe maximum amount of BaSO4 precipitated on mixing BaCl2 (0.5 M) with H2SO4 (1M) will correspond to
View SolutionThe amount of BaSO4 formed upon mixing 100mL of 20.8% BaCl2 solution with 50mL of 9.8% H2SO4 solution will be :
(Ba = 137, Cl = 35.5, S = 32, H = 1 and O =16)
View SolutionThe pH of the solution formed on mixing 20 mL of 0.05MH2SO4 with 5.0 mL of 0.45 M NaOH of 298 K is
View SolutionThe amount of heat released when 20ml,0.5M NaOHis mixed with 100ml,0.1M H2SO4 is xKJ. The heat of neutralization will be :−
View Solution

