A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with conc. H2SO4. The evolved gaseous mixture is passed through KOH pellets. Weight (in g) of the remaining product at STP will be
A
1.4
B
3.0
C
2.8
D
4.4
Text Solution
The correct Answer is:C
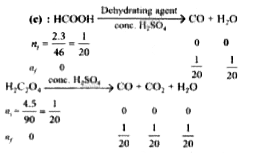
H2O is absorbed by H2SO4. Gaseous mixture (containing CO and CO2) when passed through KOH pellets, CO2 gets absorbed. Moles of CO left (unabsorbed) = 120+120=110
Mas of CO = moles × molar mass =110×25=2.8g
|
Similar Questions
Knowledge Check
When formic acid or oxalic acid is treated with conc. H2SO4 , the gas evolved is
AH2SBCOCSO2DCO2Oxalic acid on treatment with conc. H2SO4 gives
ACO onlyBCO2 onlyCCO2+H2ODH2O+CO+CO2When formic acid is heated with concentrated H2SO4, the gas evolved is
AH2SBSO2CCODCO2
Which gas is evolved when formic acid is heated with conc. H2SO4 ?
View SolutionFormic acid when treated with H2SO4 gives
View SolutionHeating of oxalic acid with conc. H2SO4 gives
View SolutionHeating of oxalic acid with conc. H2SO4 evolves
View SolutionWhen oxalic acid is dehydrated by conc. H2SO4 then it forms
View Solution

